
Write bond line formula for 2,3-dimethyl butanal.
Answer
592.2k+ views
Hint: The compound name given ends with –al meaning it is a carbonyl group is present in the compound and the main chain contains but- 4 carbon atoms. A representation of molecular structure, in which covalent bonds are represented for each degree of bond order with one side is a bond line structure.
Complete answer:
A representation of molecular structure, in which covalent bonds are represented for each degree of bond order with one side. One single bond is represented with one line, two parallel lines show a double bond, and three parallel lines show a triple bond. Carbon atom position may be indicated with letters, or may be inferred. Similar structure to, but not identical to, Lewis.
This formula is full of bonds and lines, and they sometimes end up looking like zig-zag lines because of the typical (more stable) bonds which atoms appear to make in molecules. If you're dealing with a molecular model package, it's hard to make stick straight molecules (unless they include $sp$ triple bonds) whereas zig-zag molecules and bonds are far more feasible.
- Here we are asked to make a bond line formula for the given compound.
- Firstly, let’s understand what a bond line formula is. It is a 2-D representation of molecular structure in which the bonds between the atoms are shown by lines which can represent single or multiple bonds.
- Now the bond line formula of the given compound 2,3-dimethyl butanal:
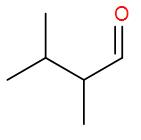 Molecular Formula : \[{{C}_{6}}{{H}_{12}}O\]
Molecular Formula : \[{{C}_{6}}{{H}_{12}}O\]
- As we can see the compound has a carbon to oxygen double bond, the functional group present is carbonyl group.
- The general formula for carbonyl compounds is ${{C}_{n}}{{H}_{2n}}O$
- We can define two families from a carbonyl group: Aldehydes and Ketones.
- As we know Aldehyde and Ketone are functional isomers.
So, bond line formula for 2,3-dimethyl butanal is
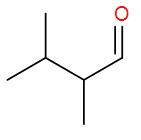
Note:
- This formula is full of bonds and lines, and they sometimes end up looking like zig-zag lines because of the typical (more stable) bonds which atoms appear to make in molecules
-$-CHO$ group is always at the end of the carbon chain, while $-CO-$ is always present in the middle of the chain.
Complete answer:
A representation of molecular structure, in which covalent bonds are represented for each degree of bond order with one side. One single bond is represented with one line, two parallel lines show a double bond, and three parallel lines show a triple bond. Carbon atom position may be indicated with letters, or may be inferred. Similar structure to, but not identical to, Lewis.
This formula is full of bonds and lines, and they sometimes end up looking like zig-zag lines because of the typical (more stable) bonds which atoms appear to make in molecules. If you're dealing with a molecular model package, it's hard to make stick straight molecules (unless they include $sp$ triple bonds) whereas zig-zag molecules and bonds are far more feasible.
- Here we are asked to make a bond line formula for the given compound.
- Firstly, let’s understand what a bond line formula is. It is a 2-D representation of molecular structure in which the bonds between the atoms are shown by lines which can represent single or multiple bonds.
- Now the bond line formula of the given compound 2,3-dimethyl butanal:

- As we can see the compound has a carbon to oxygen double bond, the functional group present is carbonyl group.
- The general formula for carbonyl compounds is ${{C}_{n}}{{H}_{2n}}O$
- We can define two families from a carbonyl group: Aldehydes and Ketones.
- As we know Aldehyde and Ketone are functional isomers.
So, bond line formula for 2,3-dimethyl butanal is

Note:
- This formula is full of bonds and lines, and they sometimes end up looking like zig-zag lines because of the typical (more stable) bonds which atoms appear to make in molecules
-$-CHO$ group is always at the end of the carbon chain, while $-CO-$ is always present in the middle of the chain.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




