
Write at least 5 compounds with the structural formula of ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}$ and identify the functional groups.
Answer
597.9k+ views
Hint: Organic compounds with similar molecular formula but different structural formula and presence of different functional groups are called Functional isomers of each other.
Complete step by step answer:
Isomerism is the phenomenon in which more than one compounds have the same chemical formula but different chemical structures.
Different structures are possible of a compound having formula ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}$. These are –
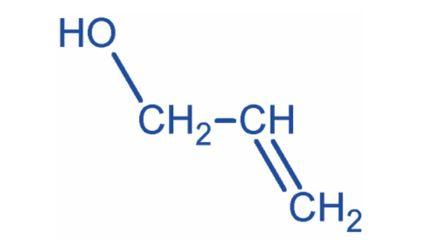
Prop-2-ene-1-ol
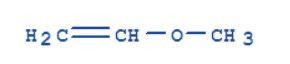
Methoxyethene
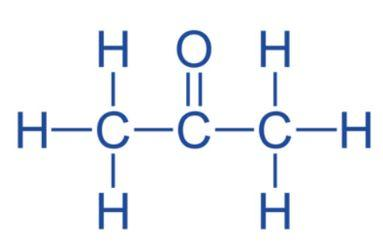
Propanone
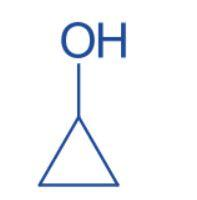
Cyclopropanol
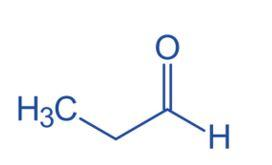
Propanal
Additional information:
When the atoms and the functional groups are joined together in different ways but the molecular formula remains the same, the type of isomerism is called Structural isomerism. There are a few types of structural isomerism:
(I) Chain Isomerism
When the carbon molecules in the chain of an organic molecule are relocated but the molecular formulas remain the same, Chain isomerism is confirmed. This alters the chain of the organic compound. The number of possible structural isomers increases greatly with the number of available atoms.
(II) Position isomerism
When compounds having the same molecular formula, same structural formula but the position of functional groups between two identical organic compounds is different, the compounds are said to show positional isomerism.
(III) Functional isomerism
Organic compounds which have the same molecular formula but the functional group present is different are called functional isomers of each other and the phenomenon is called functional isomerism.
Note: In the above given example, all compounds have same molecular formula i.e. ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}$ but have different functional groups and hence are said to be functional isomers of each other. Total 11 isomers are possible with the molecular formula ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}$.
Complete step by step answer:
Isomerism is the phenomenon in which more than one compounds have the same chemical formula but different chemical structures.
Different structures are possible of a compound having formula ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}$. These are –
Prop-2-ene-1-ol

Methoxyethene

Propanone

Cyclopropanol

Propanal

Additional information:
When the atoms and the functional groups are joined together in different ways but the molecular formula remains the same, the type of isomerism is called Structural isomerism. There are a few types of structural isomerism:
(I) Chain Isomerism
When the carbon molecules in the chain of an organic molecule are relocated but the molecular formulas remain the same, Chain isomerism is confirmed. This alters the chain of the organic compound. The number of possible structural isomers increases greatly with the number of available atoms.
(II) Position isomerism
When compounds having the same molecular formula, same structural formula but the position of functional groups between two identical organic compounds is different, the compounds are said to show positional isomerism.
(III) Functional isomerism
Organic compounds which have the same molecular formula but the functional group present is different are called functional isomers of each other and the phenomenon is called functional isomerism.
Note: In the above given example, all compounds have same molecular formula i.e. ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}$ but have different functional groups and hence are said to be functional isomers of each other. Total 11 isomers are possible with the molecular formula ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




