
Write acetylation reaction of:
A) Ethyl amine B) Diethylamine
Answer
573.9k+ views
Hint: Introduction of an acetyl group in a molecule.
Reagents used for acetylation-acetic anhydride or acetyl chloride.
Complete step by step answer:
So in the given question we have two amines ethylamine and the diethylamine, we have to write the product of two compounds after the acetylation process.
Acetylation reaction is the reaction in which an acetyl functional group or the acetoxy ($C{{H}_{3}}CO$) is introduced into a molecule. This process is called the ethanoylation as per the IUPAC nomenclature. And it can also be called as the organic esterification reaction involving the acetic acid and the final products formed are called as the acetates or the acetic esters.
For acetylation we need an acetylating reagent, to introduce an acetyl group in the chemical compounds.
The acetyl chloride is,
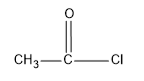
Here we use acetyl chloride for the as the acetylating reagent.
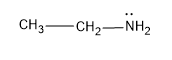
A) The first compound given here is ethylamine or ethanamine, which has a structure,
Now the ethyl amine undergoes acetylation with the acetyl chloride to yield, an amide,
N-Ethylethanamide.
And the reaction is as follows,
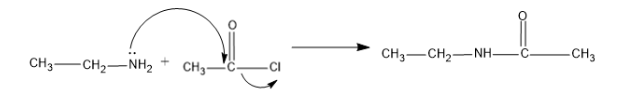
Here one hydrogen atom of amine group ($-N{{H}_{2}}$) is replaced by the acetyl group and forms the
N-Ethylethanamide.
B) The compound is the diethylamine and it has the structure,
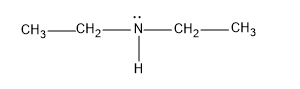
It has two ethyl groups in the two sides of the amine group, and it reacts with the acetyl chloride and gives N, N-Dimethylethanamide
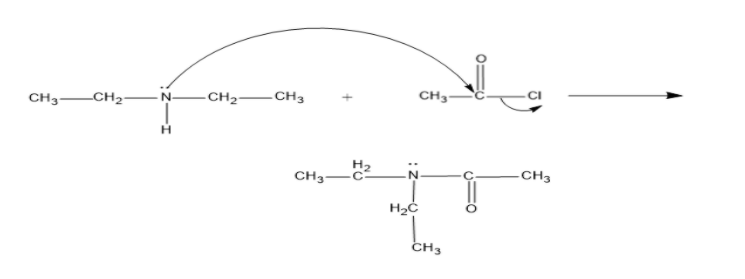
Here also one $H$ is replaced with an acetyl group.
Note: Acetic anhydride can also be used as the acetylating reagent.
Acetylation is a nucleophilic substitution reaction. In simple words, we say that acetylation reaction of amines is the removal of H atoms from the amine group and replacing it with acetyl group.
Reagents used for acetylation-acetic anhydride or acetyl chloride.
Complete step by step answer:
So in the given question we have two amines ethylamine and the diethylamine, we have to write the product of two compounds after the acetylation process.
Acetylation reaction is the reaction in which an acetyl functional group or the acetoxy ($C{{H}_{3}}CO$) is introduced into a molecule. This process is called the ethanoylation as per the IUPAC nomenclature. And it can also be called as the organic esterification reaction involving the acetic acid and the final products formed are called as the acetates or the acetic esters.
For acetylation we need an acetylating reagent, to introduce an acetyl group in the chemical compounds.
The acetyl chloride is,

Here we use acetyl chloride for the as the acetylating reagent.
A) The first compound given here is ethylamine or ethanamine, which has a structure,

Now the ethyl amine undergoes acetylation with the acetyl chloride to yield, an amide,
N-Ethylethanamide.
And the reaction is as follows,

Here one hydrogen atom of amine group ($-N{{H}_{2}}$) is replaced by the acetyl group and forms the
N-Ethylethanamide.
B) The compound is the diethylamine and it has the structure,

It has two ethyl groups in the two sides of the amine group, and it reacts with the acetyl chloride and gives N, N-Dimethylethanamide

Here also one $H$ is replaced with an acetyl group.
Note: Acetic anhydride can also be used as the acetylating reagent.
Acetylation is a nucleophilic substitution reaction. In simple words, we say that acetylation reaction of amines is the removal of H atoms from the amine group and replacing it with acetyl group.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE

Explain sex determination in humans with line diag class 12 biology CBSE




