
Write about the most common point defects.
Answer
585k+ views
Hint: The defects arising when an atom goes missing or dislocates from the crystal lattice or any atom getting added to the crystal lattice are called as point defects. These defects in the crystal lattices are centered around a point and thus, are referred to as point defects. The common point defects are:
1.Stoichiometric defects
2.Non-stoichiometric defects
3.Impurity defects
Complete step by step answer:
Step 1:
Point defects arise when an atom is missing or occupies an irregular place in the crystal lattice. These irregularities are centered around a point and thus, they are known as point defects.
The point defects arise due to the following reasons:
1.Displacement of an atom in the crystal lattice.
2.Addition of an extra atom in the crystal lattice.
3.Missing atom in the crystal lattice.
Step 2:
There are three types of common point defects as follows:
1.Stoichiometric Defects:
In stoichiometric defects, the ratio of cations and anions does not change.
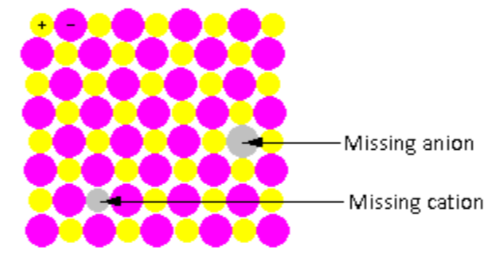
In the crystal lattice shown in the figure, one cation and one anion are missing. Thus, the ratio of cations and anion remains the same even after the defect. Such point defects are known as stoichiometric defects.
2.Non-Stoichiometric Defects:
In non-stoichiometric defects, the ratio of cations and anions changes.
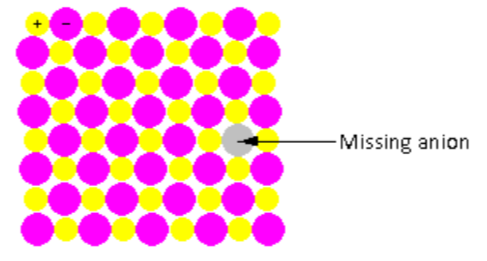
In the crystal lattice shown in the figure, one anion is missing. Thus, the ratio of cations and anions changes after the defect. Such point defects are known as non-stoichiometric defects.
3.Impurity Defects:
In impurity defects, a foreign atom gets added to the crystal lattice as an impurity.
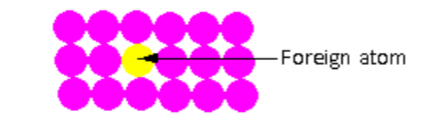
In the crystal lattice shown in the figure, one foreign atom is introduced in the crystal lattice. The foreign atom introduced is known as the impurity. Such point defects are known as impurity defects.
Note:
The stoichiometric and non-stoichiometric defects arise due to the displacement of an atom from the crystal lattice. The impurity defect arises due to addition of an extra atom in the crystal lattice.
The defects in the lattice arise during the crystallization of the solids. A large number of small crystals are formed in the process of crystallization. There are two types of defects based on the location of the defects.
1.Point defects: Point defects are centered around a point.
2.Line defects: Line defects occur along a line instead of a point.
1.Stoichiometric defects
2.Non-stoichiometric defects
3.Impurity defects
Complete step by step answer:
Step 1:
Point defects arise when an atom is missing or occupies an irregular place in the crystal lattice. These irregularities are centered around a point and thus, they are known as point defects.
The point defects arise due to the following reasons:
1.Displacement of an atom in the crystal lattice.
2.Addition of an extra atom in the crystal lattice.
3.Missing atom in the crystal lattice.
Step 2:
There are three types of common point defects as follows:
1.Stoichiometric Defects:
In stoichiometric defects, the ratio of cations and anions does not change.

In the crystal lattice shown in the figure, one cation and one anion are missing. Thus, the ratio of cations and anion remains the same even after the defect. Such point defects are known as stoichiometric defects.
2.Non-Stoichiometric Defects:
In non-stoichiometric defects, the ratio of cations and anions changes.

In the crystal lattice shown in the figure, one anion is missing. Thus, the ratio of cations and anions changes after the defect. Such point defects are known as non-stoichiometric defects.
3.Impurity Defects:
In impurity defects, a foreign atom gets added to the crystal lattice as an impurity.

In the crystal lattice shown in the figure, one foreign atom is introduced in the crystal lattice. The foreign atom introduced is known as the impurity. Such point defects are known as impurity defects.
Note:
The stoichiometric and non-stoichiometric defects arise due to the displacement of an atom from the crystal lattice. The impurity defect arises due to addition of an extra atom in the crystal lattice.
The defects in the lattice arise during the crystallization of the solids. A large number of small crystals are formed in the process of crystallization. There are two types of defects based on the location of the defects.
1.Point defects: Point defects are centered around a point.
2.Line defects: Line defects occur along a line instead of a point.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




