
Write a short note on Zwitterions.
Answer
582.9k+ views
Hint: Zwitterion , also known as the dipolar ion is formed in amino acids due to the transfer of the proton from the carboxylic acid group to the amino group and in this, the acidic nature is due to ammonium ion and basic nature is due to carboxylate ion.
Complete step by step answer:
Amino acids are the organic compounds containing both the amino group and carboxylic group in their molecules. They are represented by the general formula as:
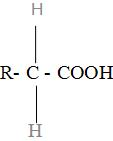
Here, -C- is called as alpha carbon because the carbon atom attached next to the a=carboxyl group is called as alpha carbon.
R is the side chain and is different for different amino acids.
-COOH is the carboxylic group.
$-\text{N}{{\text{H}}_{2}}$ is the amino group.
In the amino acid , the acidic properties are due to the $-\text{NH}_{3}^{+}$ group and the basic properties are due to the $\text{-CO}{{\text{O}}^{-}}$. This is so because the proton from the carboxylic group shifts to the amino group to produce dipolar ions. Such a dipolar ion is called the inner salt or the zwitterion.
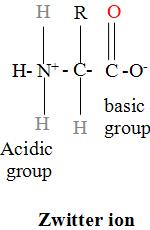
Zwitterions are non-volatile crystalline solids having high melting and boiling points. They are quite soluble in the polar solvents like the water but are insoluble in non-polar solvents like benzene and ether and have high dipole moments too.
Note: Amino acids have exceptionally low values of ${{\text{K}}_{a}}$ and ${{\text{K}}_{b}}$ as compared to the values for the carboxylic acids and aliphatic amines. Their low values are due to the zwitterion because according to it, the ammonium ion is an acidic center while a carboxylate ion is a basic center in amino acids and are responsible for the acidic and basic character.
Complete step by step answer:
Amino acids are the organic compounds containing both the amino group and carboxylic group in their molecules. They are represented by the general formula as:
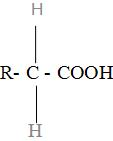
Here, -C- is called as alpha carbon because the carbon atom attached next to the a=carboxyl group is called as alpha carbon.
R is the side chain and is different for different amino acids.
-COOH is the carboxylic group.
$-\text{N}{{\text{H}}_{2}}$ is the amino group.
In the amino acid , the acidic properties are due to the $-\text{NH}_{3}^{+}$ group and the basic properties are due to the $\text{-CO}{{\text{O}}^{-}}$. This is so because the proton from the carboxylic group shifts to the amino group to produce dipolar ions. Such a dipolar ion is called the inner salt or the zwitterion.
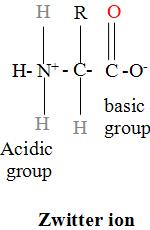
Zwitterions are non-volatile crystalline solids having high melting and boiling points. They are quite soluble in the polar solvents like the water but are insoluble in non-polar solvents like benzene and ether and have high dipole moments too.
Note: Amino acids have exceptionally low values of ${{\text{K}}_{a}}$ and ${{\text{K}}_{b}}$ as compared to the values for the carboxylic acids and aliphatic amines. Their low values are due to the zwitterion because according to it, the ammonium ion is an acidic center while a carboxylate ion is a basic center in amino acids and are responsible for the acidic and basic character.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




