
Write a reaction that indicates presence of the aldehyde group in glucose.
Answer
569.4k+ views
Hint: Glucose is defined as an organic compound that contains six carbon atoms and one aldehyde group. It is a monosaccharide that can exist in a closed ring as well as an open chain. It occurs naturally in fruits and other parts of plants. In this question, we will see a reaction of glucose having an aldehyde group and hydroxyl amine.
Complete step by step answer:
Glucose is defined as a sugar having molecular formula ${{C}_{6}}{{H}_{12}}{{O}_{6}}$ . $D-$ glucose occurs naturally whereas $L-$ glucose is made synthetically. The $D-$ glucose is also called dextrose.
Let us see the reaction which indicates the presence of the aldehyde group in glucose.
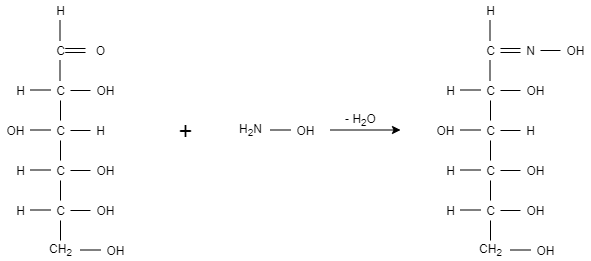
When glucose reacts with hydroxylamine, it results in the formation of glucose oxime. The glucose oxime is formed when water is removed from both the compounds. Oxygen is removed from carbonyl containing glucose and hydrogen is removed from hydroxylamine. Only the aldehyde group of glucose is reacting therefore it indicates the presence of the aldehyde group in glucose.
Additional information:
Hydroxylamine is defined as an inorganic compound that contains a molecular formula $N{{H}_{2}}OH$ . It is a white, crystalline pure material. Hydroxyl amine is used as a reducing agent and can also act as a reducing agent and can also act as antioxidants for fatty acids. On heating, hydroxyl amine may explode.
Glucose is used as aldohexose in living organisms. Glucose contains less tendency than any other aldohexoses to react non – specifically with amine group of proteins
Note: Glucose is defined as the group of carbohydrates which is also known as simple sugars.
Glucose contains six carbon atoms in which the first carbon is a part of an aldehyde group and each of the other five carbons have one hydroxyl group. The remaining bonds of the carbon atoms have hydrogen atoms.
Through photosynthesis, glucose is produced by the plants using sunlight and water. Glucose is also used by living organisms as an energy source.
Complete step by step answer:
Glucose is defined as a sugar having molecular formula ${{C}_{6}}{{H}_{12}}{{O}_{6}}$ . $D-$ glucose occurs naturally whereas $L-$ glucose is made synthetically. The $D-$ glucose is also called dextrose.
Let us see the reaction which indicates the presence of the aldehyde group in glucose.

When glucose reacts with hydroxylamine, it results in the formation of glucose oxime. The glucose oxime is formed when water is removed from both the compounds. Oxygen is removed from carbonyl containing glucose and hydrogen is removed from hydroxylamine. Only the aldehyde group of glucose is reacting therefore it indicates the presence of the aldehyde group in glucose.
Additional information:
Hydroxylamine is defined as an inorganic compound that contains a molecular formula $N{{H}_{2}}OH$ . It is a white, crystalline pure material. Hydroxyl amine is used as a reducing agent and can also act as a reducing agent and can also act as antioxidants for fatty acids. On heating, hydroxyl amine may explode.
Glucose is used as aldohexose in living organisms. Glucose contains less tendency than any other aldohexoses to react non – specifically with amine group of proteins
Note: Glucose is defined as the group of carbohydrates which is also known as simple sugars.
Glucose contains six carbon atoms in which the first carbon is a part of an aldehyde group and each of the other five carbons have one hydroxyl group. The remaining bonds of the carbon atoms have hydrogen atoms.
Through photosynthesis, glucose is produced by the plants using sunlight and water. Glucose is also used by living organisms as an energy source.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




