
What word or two-word phrase describes the shape of the formaldehyde ($C{{H}_{2}}O$) molecule?
Answer
527.4k+ views
Hint: Formaldehyde is a compound having a carbonyl group ($>C=O$) as a functional group. The carbon atom is a tetravalent atom, so it can form four covalent bonds, so the atoms attached in the formaldehyde will decide the shape.
Complete answer:
The shape of any molecule depends on the number and type of atoms attached to the central carbon atom.
Mostly if there are only two atoms in the molecule then it will be a linear structure, if three atoms are present on the central atom then it will be trigonal planar, if four atoms are present on the central atom then the structure will be tetrahedral or square planar, etc.
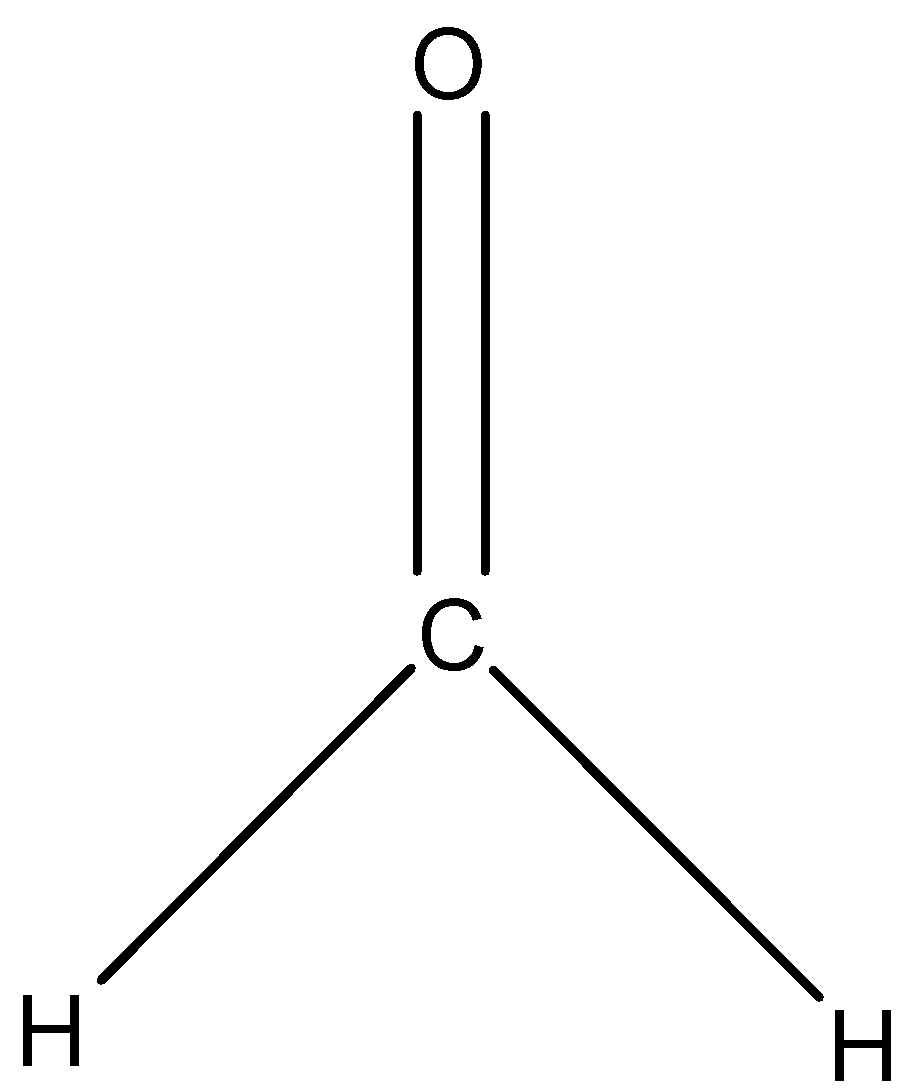
The given compound in the question is formaldehyde whose formula is $C{{H}_{2}}O$. The carbon atom will be the central atom in the molecule. The carbon atom is a tetravalent atom so, it can form four covalent bonds, so the atoms attached in the formaldehyde will decide the shape.
So, there is one oxygen atom and two hydrogen atoms attached to it. We know that an oxygen atom needs only two electrons to complete its octet, so it will form a double bond with the carbon atom. Each hydrogen atom will form a single bond with the carbon atom. So, the shape of the formaldehyde molecule will be trigonal planar. The structure is given below:
Note:
Don't get confused that all the bonds are present around the carbon atom so, its shape should be tetrahedral because a double bond is formed by oxygen which will decrease one position of the substituent.
Complete answer:
The shape of any molecule depends on the number and type of atoms attached to the central carbon atom.
Mostly if there are only two atoms in the molecule then it will be a linear structure, if three atoms are present on the central atom then it will be trigonal planar, if four atoms are present on the central atom then the structure will be tetrahedral or square planar, etc.
The given compound in the question is formaldehyde whose formula is $C{{H}_{2}}O$. The carbon atom will be the central atom in the molecule. The carbon atom is a tetravalent atom so, it can form four covalent bonds, so the atoms attached in the formaldehyde will decide the shape.
So, there is one oxygen atom and two hydrogen atoms attached to it. We know that an oxygen atom needs only two electrons to complete its octet, so it will form a double bond with the carbon atom. Each hydrogen atom will form a single bond with the carbon atom. So, the shape of the formaldehyde molecule will be trigonal planar. The structure is given below:

Note:
Don't get confused that all the bonds are present around the carbon atom so, its shape should be tetrahedral because a double bond is formed by oxygen which will decrease one position of the substituent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




