
Williamson's synthesis is used to prepare
(A) Diethyl ether
(B) PVC
(C) Bakelite
(D) Ethyl alcohol
Answer
501k+ views
Hint: The Williamson ether synthesis is an organic process in which an organohalide and a deprotonated alcohol are combined to create an Ether (alkoxide). Alexander Williamson invented this reaction in 1850. It usually includes a $ {S_N}2 $ reaction between an alkoxide ion and a primary Alkyl halide. This reaction is significant in organic chemistry since it contributed to the discovery of the structure of ethers.
Complete answer:
The following is the general response mechanism: $ {\left[ {Na} \right]^ + }{[{C_2}{H_5}O]^ - }\; + {\text{ }}{C_2}{H_5}Cl{\text{ }} \to {\text{ }}{C_2}{H_5}O{C_2}{H_5}\; + {\text{ }}{\left[ {Na} \right]^ + }{\left[ {Cl} \right]^ - } $
An $ {S_N}2 $ bimolecular nucleophilic substitution mechanism is used in the Williamson ether synthesis. A backside assault of an electrophile by a nucleophile occurs in an $ {S_N}2 $ reaction mechanism, and it occurs in a coordinated manner (happens all at once). A suitable leaving group that is highly electronegative, such as a halide, is required for the $ {S_N}2 $ reaction to occur. An alkoxide ion ( $ R{O^ - } $ ) functions as the nucleophile in the Williamson ether reaction, attacking the electrophilic carbon with the leaving group, which is usually an alkyl tosylate or an alkyl halide. Because secondary and tertiary leaving sites prefer to continue as an elimination reaction, the leaving site must be a primary carbon. Due to steric hindrance, this reaction does not promote the synthesis of bulky ethers like di-tert butyl ether, and instead prefers the formation of alkenes.
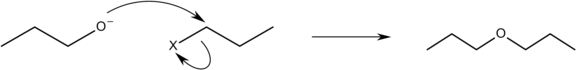
The Williamson reaction has a wide range of applications, is frequently employed in both laboratory and commercial synthesis, and is still the most straightforward and widely utilised way of producing ethers. It's simple to make both symmetrical and asymmetrical ethers. Epoxides are produced by the intramolecular interaction of halohydrins in particular. When it comes to unsymmetrical ethers, there are two options for reactant selection, and one is generally preferred based on availability or reactivity. The Williamson reaction is also commonly employed to make an ether from two alcohols indirectly. After converting one of the alcohols to a leaving group (typically tosylate), the two are reacted together.
Therefore, Williamson's synthesis is used to prepare Diethyl ether. So, option (A) is correct.
Note:
The Williamson reaction frequently competes with base-catalyzed alkylating agent elimination, and the type of the leaving group, as well as reaction circumstances (especially temperature and solvent), can have a significant impact on which is preferred. Some alkylating agent structures, in particular, can be particularly susceptible to removal. The Williamson reaction can compete with alkylation on the ring when the nucleophile is an aryloxide ion because the aryloxide is an ambident nucleophile.
Complete answer:
The following is the general response mechanism: $ {\left[ {Na} \right]^ + }{[{C_2}{H_5}O]^ - }\; + {\text{ }}{C_2}{H_5}Cl{\text{ }} \to {\text{ }}{C_2}{H_5}O{C_2}{H_5}\; + {\text{ }}{\left[ {Na} \right]^ + }{\left[ {Cl} \right]^ - } $
An $ {S_N}2 $ bimolecular nucleophilic substitution mechanism is used in the Williamson ether synthesis. A backside assault of an electrophile by a nucleophile occurs in an $ {S_N}2 $ reaction mechanism, and it occurs in a coordinated manner (happens all at once). A suitable leaving group that is highly electronegative, such as a halide, is required for the $ {S_N}2 $ reaction to occur. An alkoxide ion ( $ R{O^ - } $ ) functions as the nucleophile in the Williamson ether reaction, attacking the electrophilic carbon with the leaving group, which is usually an alkyl tosylate or an alkyl halide. Because secondary and tertiary leaving sites prefer to continue as an elimination reaction, the leaving site must be a primary carbon. Due to steric hindrance, this reaction does not promote the synthesis of bulky ethers like di-tert butyl ether, and instead prefers the formation of alkenes.
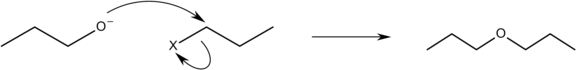
The Williamson reaction has a wide range of applications, is frequently employed in both laboratory and commercial synthesis, and is still the most straightforward and widely utilised way of producing ethers. It's simple to make both symmetrical and asymmetrical ethers. Epoxides are produced by the intramolecular interaction of halohydrins in particular. When it comes to unsymmetrical ethers, there are two options for reactant selection, and one is generally preferred based on availability or reactivity. The Williamson reaction is also commonly employed to make an ether from two alcohols indirectly. After converting one of the alcohols to a leaving group (typically tosylate), the two are reacted together.
Therefore, Williamson's synthesis is used to prepare Diethyl ether. So, option (A) is correct.
Note:
The Williamson reaction frequently competes with base-catalyzed alkylating agent elimination, and the type of the leaving group, as well as reaction circumstances (especially temperature and solvent), can have a significant impact on which is preferred. Some alkylating agent structures, in particular, can be particularly susceptible to removal. The Williamson reaction can compete with alkylation on the ring when the nucleophile is an aryloxide ion because the aryloxide is an ambident nucleophile.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




