
Wilkinson catalyst is:
A.$\left[ {{{\left( {P{h_3}P} \right)}_3}RhCl} \right]\left( {Et = {C_2}{H_5}} \right)$
B.$\left[ {{{\left( {E{t_3}P} \right)}_3}IrCl} \right]$
C.$\left[ {{{\left( {E{t_3}P} \right)}_3}RhCl} \right]$
D.$\left[ {{{\left( {P{h_3}P} \right)}_3}IrCl} \right]$
Answer
566.7k+ views
Hint: We need to remember that a substance is added to a chemical reaction which alters the reaction without getting consumed in the process is called the catalyst. Catalyst, increased the rate of the reaction or decreased the rate of the reaction. Small amount of catalyst is required to alter the chemical reaction. The word catalyst is derived from Greek, meaning to unite or to pick up.
Complete answer:
Sir Geoffrey Wilkinson was an English chemist. The Wilkinson catalyst is called after the chemist Wilkinson and the catalyst is mostly used catalyst, it is an organometallic compound.
We must know that the rhodium complex is used in the Wilkinson catalyst. $chlorido\left( {triphenylphosphine} \right)rhodium(I)$ is the common name for the Wilkinson catalyst and the molecular formula is $\left[ {{{\left( {P{h_3}P} \right)}_3}RhCl} \right]$ . $ph$ is phenyl group and $P$ is a phosphine ligand.
Option A. $\left[ {{{\left( {P{h_3}P} \right)}_3}RhCl} \right]$ is the correct option.
We can prepare the Wilkinson catalyst by the reaction of hydrated rhodium chloride with excess triphenylphosphine. We can write the chemical equation for this chemical reaction as, $RhC{l_3}{\left( {{H_2}O} \right)_3} + 4PP{h_3} \to RhCl{\left( {PP{h_3}} \right)_3} + 2HCl + 2{H_2}O$
We can draw the structure of Wilkinson Catalyst as,
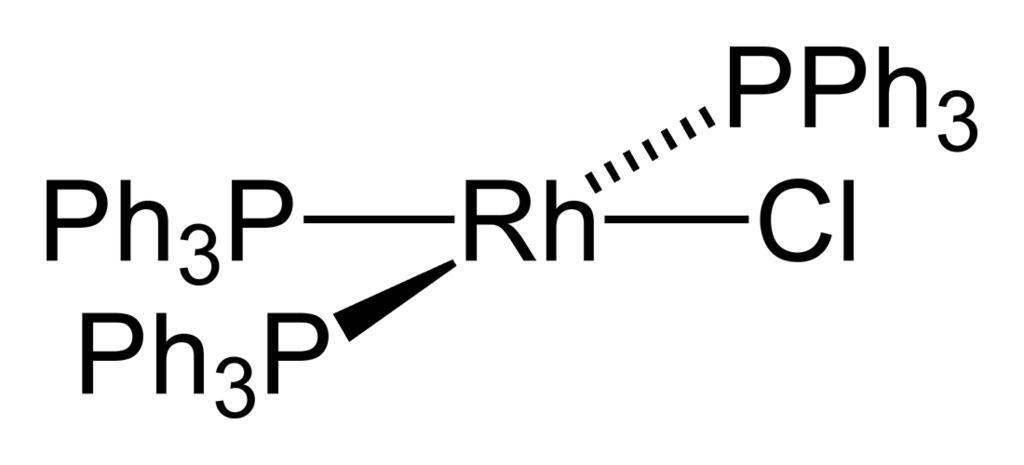
Therefore, the option A is the correct answer.
Additional information:
We have to know that Wilkinson’s catalyst is a solid and the color is red-brown. In hydrocarbon solvents (such as benzene and THF) Wilkinson catalyst is soluble and also soluble in dichloromethane. Wilkinson catalysts also participate in non-catalytic processes. Hydrogenation of olefin is carried out successfully by Wilkinson catalyst and it is an effective homogeneous catalyst.
Note:
We need to know that this particular catalyst was discovered independently by two groups and this often happens in discoveries. This is universally referred to as the Wilkinson catalyst. In pharmaceutical industries Wilkinson catalyst is an important catalyst for making specific drugs, in spite of the high cost of the catalyst. The ligand bulky phosphine plays an important role for making the selective complex. It is also used in the selective hydrogenation of $C = C$ bonds, which are not sterically hindered.
Complete answer:
Sir Geoffrey Wilkinson was an English chemist. The Wilkinson catalyst is called after the chemist Wilkinson and the catalyst is mostly used catalyst, it is an organometallic compound.
We must know that the rhodium complex is used in the Wilkinson catalyst. $chlorido\left( {triphenylphosphine} \right)rhodium(I)$ is the common name for the Wilkinson catalyst and the molecular formula is $\left[ {{{\left( {P{h_3}P} \right)}_3}RhCl} \right]$ . $ph$ is phenyl group and $P$ is a phosphine ligand.
Option A. $\left[ {{{\left( {P{h_3}P} \right)}_3}RhCl} \right]$ is the correct option.
We can prepare the Wilkinson catalyst by the reaction of hydrated rhodium chloride with excess triphenylphosphine. We can write the chemical equation for this chemical reaction as, $RhC{l_3}{\left( {{H_2}O} \right)_3} + 4PP{h_3} \to RhCl{\left( {PP{h_3}} \right)_3} + 2HCl + 2{H_2}O$
We can draw the structure of Wilkinson Catalyst as,

Therefore, the option A is the correct answer.
Additional information:
We have to know that Wilkinson’s catalyst is a solid and the color is red-brown. In hydrocarbon solvents (such as benzene and THF) Wilkinson catalyst is soluble and also soluble in dichloromethane. Wilkinson catalysts also participate in non-catalytic processes. Hydrogenation of olefin is carried out successfully by Wilkinson catalyst and it is an effective homogeneous catalyst.
Note:
We need to know that this particular catalyst was discovered independently by two groups and this often happens in discoveries. This is universally referred to as the Wilkinson catalyst. In pharmaceutical industries Wilkinson catalyst is an important catalyst for making specific drugs, in spite of the high cost of the catalyst. The ligand bulky phosphine plays an important role for making the selective complex. It is also used in the selective hydrogenation of $C = C$ bonds, which are not sterically hindered.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




