
Why is \[Pb{{O}_{2}}\] not peroxide?
Answer
507.3k+ views
Hint: Peroxide ion has the molecular formula \[{{O}_{2}}^{2-}\]. The treatment of metallic oxides with acid i.e. hydrochloric acid produces hydrogen peroxide. The formation of peroxides is feasible with hydrogen, alkali and alkaline earth metals.
Complete answer:
Peroxides are formed by the treatment of metallic oxides with acid i.e. hydrochloric acid. The molecular formula of peroxide ion is \[{{O}_{2}}^{2-}\]. The structure of hydrogen peroxide is given in the following diagram:
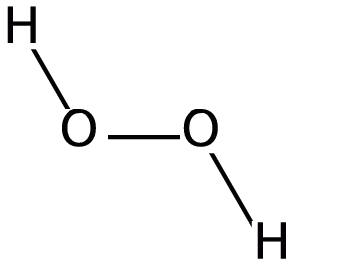
From the above diagram, we conclude that in peroxides, there is a single bond present between two oxygen atoms and charge on each oxygen atom is \[-1\]. Let us now consider the structure of \[Pb{{O}_{2}}\] which is described in the following diagram-
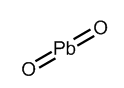
From the above structure, we conclude that \[O-O\]bond is not present in \[Pb{{O}_{2}}\] . In \[Pb{{O}_{2}}\] , lead is present in \[+4\] oxidation state while each oxygen atom carries a \[-2\] charge.
Therefore, \[Pb{{O}_{2}}\] is not a peroxide.
Additional information:
The chemical name of \[Pb{{O}_{2}}\] is lead dioxide. The electronic configuration of lead is \[[Xe]6{{s}^{2}}4{{f}^{14}}5{{d}^{10}}6{{p}^{2}}\]. Considering the electronic configuration into the account, the oxidation state of lead should be \[+2\] and \[+4\]. Due to the inert pair effect, \[+2\]oxidation state of lead is more stable in comparison to \[+4\] . As a consequence, \[PbO\] is much more stable than \[Pb{{O}_{2}}\].
Note:
It is important to note that the structure of \[Pb{{O}_{2}}\] doesn’t contain \[O-O\] bond. Hence, \[Pb{{O}_{2}}\] is not a peroxide. In \[Pb{{O}_{2}}\] , lead is present in \[+4\] oxidation state while each oxygen atom carries a \[-2\] charge.
Complete answer:
Peroxides are formed by the treatment of metallic oxides with acid i.e. hydrochloric acid. The molecular formula of peroxide ion is \[{{O}_{2}}^{2-}\]. The structure of hydrogen peroxide is given in the following diagram:

From the above diagram, we conclude that in peroxides, there is a single bond present between two oxygen atoms and charge on each oxygen atom is \[-1\]. Let us now consider the structure of \[Pb{{O}_{2}}\] which is described in the following diagram-

From the above structure, we conclude that \[O-O\]bond is not present in \[Pb{{O}_{2}}\] . In \[Pb{{O}_{2}}\] , lead is present in \[+4\] oxidation state while each oxygen atom carries a \[-2\] charge.
Therefore, \[Pb{{O}_{2}}\] is not a peroxide.
Additional information:
The chemical name of \[Pb{{O}_{2}}\] is lead dioxide. The electronic configuration of lead is \[[Xe]6{{s}^{2}}4{{f}^{14}}5{{d}^{10}}6{{p}^{2}}\]. Considering the electronic configuration into the account, the oxidation state of lead should be \[+2\] and \[+4\]. Due to the inert pair effect, \[+2\]oxidation state of lead is more stable in comparison to \[+4\] . As a consequence, \[PbO\] is much more stable than \[Pb{{O}_{2}}\].
Note:
It is important to note that the structure of \[Pb{{O}_{2}}\] doesn’t contain \[O-O\] bond. Hence, \[Pb{{O}_{2}}\] is not a peroxide. In \[Pb{{O}_{2}}\] , lead is present in \[+4\] oxidation state while each oxygen atom carries a \[-2\] charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




