
Why is $ NO_3^ - $ a nonpolar molecule?
Answer
481.2k+ views
Hint: The molecules are said to be polar when there is a difference in electronegativity present between the bonded atoms. Non-polar molecules are those where the electrons are shared equally between the atoms across a bond or when the polar bonds tend to cancel out each other.
Complete Step By Step Answer:
The ion given to us is the Nitrate ion. First let us find the hybridisation, shape and geometry of the molecule. The hybridisation of polyatomic compounds can be given by the formula:
$ Hybridisation = \dfrac{{no.of{\text{ }}valence{\text{ }}electron\operatorname{s} {\text{ }}in{\text{ }}the{\text{ }}central{\text{ }}atom + no.ofHydrogen{\text{ }}Atoms + no.of{\text{ }}Halide{\text{ }}Atoms \pm Formal{\text{ }}Ch\arg e}}{2} $
If the answer obtained is:
The hybridisation of the Nitrate ion will be $ = \dfrac{{5 + 1}}{2} = \dfrac{6}{2} = 3 \to s{p^2} $
The geometry and of the molecule will be trigonal planar with zero lone pair and can be given as:
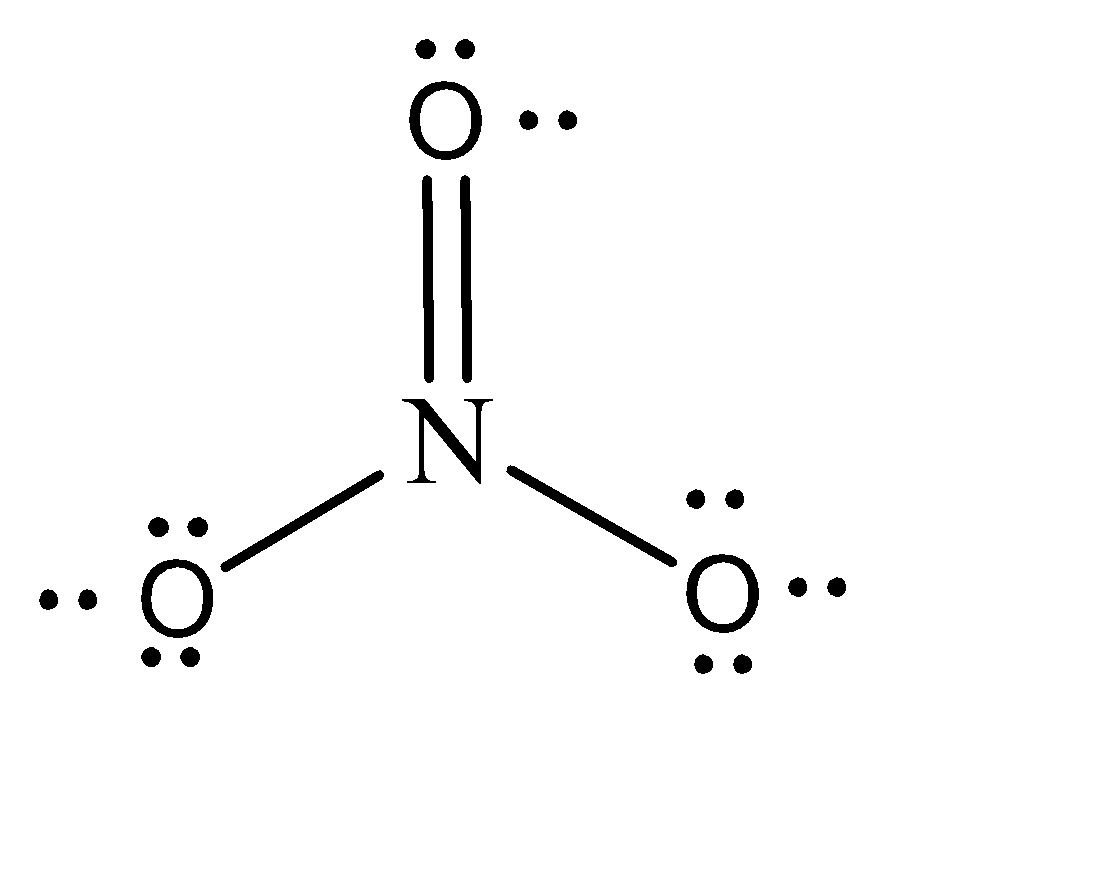
$ NO_3^ - $ is a stable molecule because of the presence of one double bond between the N-O. The polarity of the molecule is always misinterpreted because of the presence of this double. Even after having a double bond and covalent sharing of electrons, it is a Nonpolar molecule. This expectio is explained as follows:
$ NO_3^ - $ has a symmetrical structure of trigonal planar, irrespective of the double bond being present. This cancels the dipole moment present inside the molecule. Since there is no net dipole moment present, there will be no separation of charges between the two ends of the bond, and hence no polarity will be generated. Therefore, $ NO_3^ - $ is a nonpolar molecule.
Note:
The three N-O bonds are actually identical. Due to delocalisation of electrons each oxygen atom carries a formal charge of -2/3 and the nitrogen has +1 charge. The overall charge on the atom hence can be given as $ (3 \times - 2/3) + 1 = - 1 $ . This is the overall charge on the nitrate ion.
Complete Step By Step Answer:
The ion given to us is the Nitrate ion. First let us find the hybridisation, shape and geometry of the molecule. The hybridisation of polyatomic compounds can be given by the formula:
$ Hybridisation = \dfrac{{no.of{\text{ }}valence{\text{ }}electron\operatorname{s} {\text{ }}in{\text{ }}the{\text{ }}central{\text{ }}atom + no.ofHydrogen{\text{ }}Atoms + no.of{\text{ }}Halide{\text{ }}Atoms \pm Formal{\text{ }}Ch\arg e}}{2} $
If the answer obtained is:
| Answer Obtained/Steric Number | Hybridisation | Geometry |
| 2 | sp | Linear |
| 3 | $ s{p^2} $ | Trigonal Planar |
| 4 | $ s{p^3} $ | Tetrahedral |
| 5 | $ s{p^3}d $ | Trigonal Bipyramidal |
| 6 | $ s{p^3}{d^2} $ | Octahedral |
The hybridisation of the Nitrate ion will be $ = \dfrac{{5 + 1}}{2} = \dfrac{6}{2} = 3 \to s{p^2} $
The geometry and of the molecule will be trigonal planar with zero lone pair and can be given as:

$ NO_3^ - $ is a stable molecule because of the presence of one double bond between the N-O. The polarity of the molecule is always misinterpreted because of the presence of this double. Even after having a double bond and covalent sharing of electrons, it is a Nonpolar molecule. This expectio is explained as follows:
$ NO_3^ - $ has a symmetrical structure of trigonal planar, irrespective of the double bond being present. This cancels the dipole moment present inside the molecule. Since there is no net dipole moment present, there will be no separation of charges between the two ends of the bond, and hence no polarity will be generated. Therefore, $ NO_3^ - $ is a nonpolar molecule.
Note:
The three N-O bonds are actually identical. Due to delocalisation of electrons each oxygen atom carries a formal charge of -2/3 and the nitrogen has +1 charge. The overall charge on the atom hence can be given as $ (3 \times - 2/3) + 1 = - 1 $ . This is the overall charge on the nitrate ion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




