
Why is bithional added to soap?
Answer
588.6k+ views
Hint: Soaps are used as cleansing agents, and bithional is added to soap. Bithional exhibits specific kinds of properties like killing action. Now, the reason can be identified for its addition to soaps.
Complete step by step answer:
First, let us know about the soaps. As mentioned, these are used as cleansing agents. It is a chemical mixture of sodium salt, or potassium salt, and shows their cleansing action in water.
-As we know, the soaps also contain fatty acids, i.e. fats, and oils. The examples of soaps are sodium stearate, sodium palmitate, and so on.
-Thus, the fats, and oils are extracted from the various plants, and animals that are required for the production of soaps.
-We can say while the production of soaps, alkalis like sodium hydroxide, and potassium hydroxide are also used, and these are composed clinically.
-Now, if we talk about the addition of bithional to soaps. Then, we can say that bithional exhibits the properties like antibacterial, and algaecide, and it is an aromatic compound
So, we can say that bithional will impart antiseptic property.
-There is some kind of odour produced on bacterial decomposition of organic matter into skin, and it is also reduced with the addition of bithional.
-In the last, we can conclude that bithional is added to soaps because of its antiseptic property.
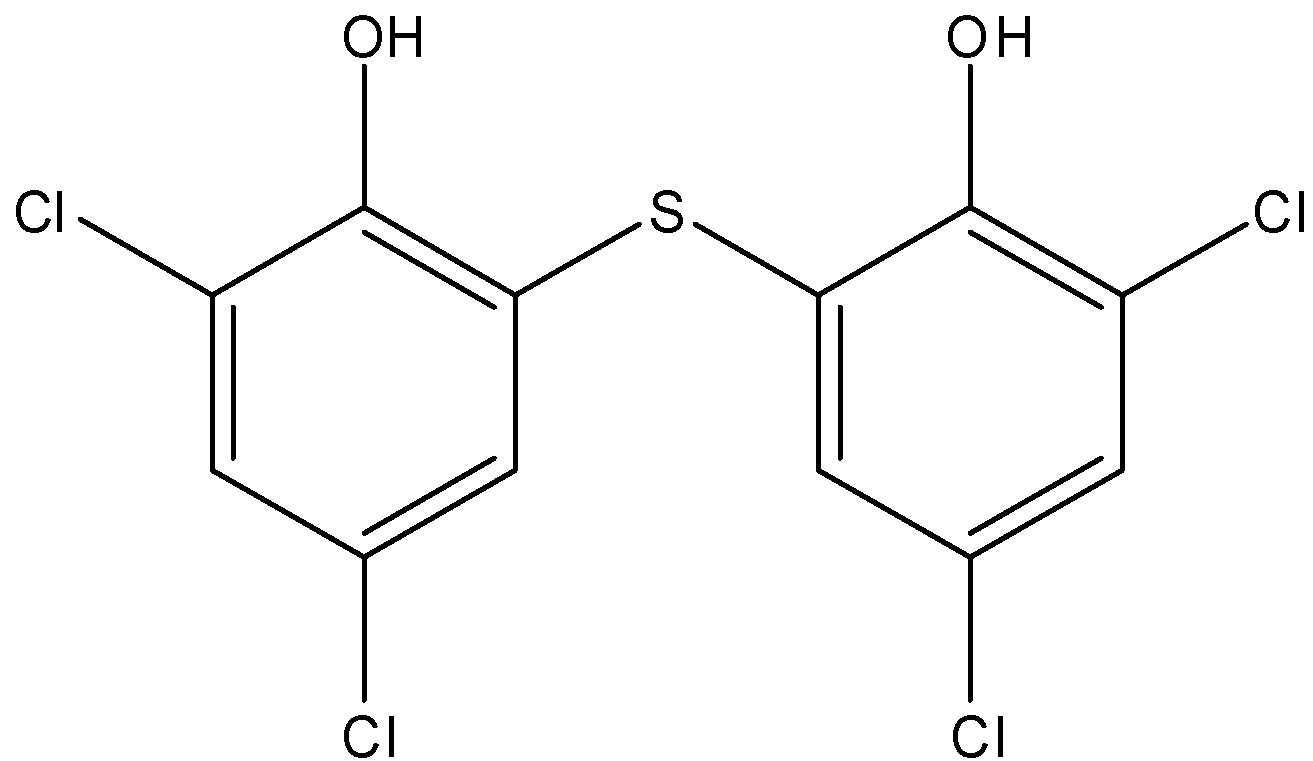
Note: It should be noted that bithional is an aromatic compound whose IUPAC name is 2,4-dichloro-6-(3,5-dichloro-2-hydroxyphenyl)sulfonyl phenol. The structure of the compound is shown below-
Complete step by step answer:
First, let us know about the soaps. As mentioned, these are used as cleansing agents. It is a chemical mixture of sodium salt, or potassium salt, and shows their cleansing action in water.
-As we know, the soaps also contain fatty acids, i.e. fats, and oils. The examples of soaps are sodium stearate, sodium palmitate, and so on.
-Thus, the fats, and oils are extracted from the various plants, and animals that are required for the production of soaps.
-We can say while the production of soaps, alkalis like sodium hydroxide, and potassium hydroxide are also used, and these are composed clinically.
-Now, if we talk about the addition of bithional to soaps. Then, we can say that bithional exhibits the properties like antibacterial, and algaecide, and it is an aromatic compound
So, we can say that bithional will impart antiseptic property.
-There is some kind of odour produced on bacterial decomposition of organic matter into skin, and it is also reduced with the addition of bithional.
-In the last, we can conclude that bithional is added to soaps because of its antiseptic property.
Note: It should be noted that bithional is an aromatic compound whose IUPAC name is 2,4-dichloro-6-(3,5-dichloro-2-hydroxyphenyl)sulfonyl phenol. The structure of the compound is shown below-

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




