
White fumes are:
(A) Chlorine
(B) Hydrogen sulphide
(C) Ammonium chloride
(D) Ammonium hydroxide

Answer
541.5k+ views
Hint: Knowing the compound at the place of ‘A’ can help us solve the given equations easily and thus, find the answer.
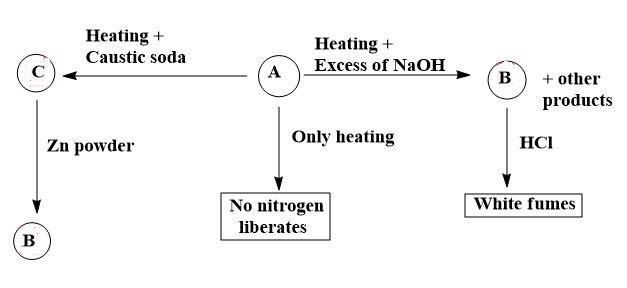
As, we need to find only the composition of white fumes, we can solve only one side of the given equation. But for better understanding we should simplify each side of the reaction given (as finding ‘B’ would be simpler from the other path as $A\to C\to B$ )
Complete answer:
Let us move towards the given sample of equation to simplify it and find the required answer.
By analysing and having basic knowledge, let us say that the compound ‘A’ is ammonium nitrate i.e. $N{{H}_{4}}N{{O}_{3}}$ . Now, by moving step by step;
1. On thermal decomposition i.e. heating ammonium nitrate, it gives a mixture of ${{N}_{2}}O+{{H}_{2}}O$ as,
$N{{H}_{4}}N{{O}_{3}}\to {{N}_{2}}O+2{{H}_{2}}O$
2. On heating ammonium nitrate with caustic soda, it gives a mixture of $NaN{{O}_{3}}+N{{H}_{3}}+{{H}_{2}}O$ as,
$N{{H}_{4}}N{{O}_{3}}+NaOH\to NaN{{O}_{3}}+N{{H}_{3}}+{{H}_{2}}O$
Then the mixture of $NaN{{O}_{3}}+N{{H}_{3}}+{{H}_{2}}O$ on reduction with Zn powder gives a gas which is a replacement of ‘B’ i.e. ammonia gas.
3. On heating ammonium nitrate with excess NaOH, it gives ‘B’ i.e. ammonia gas along with other products. When the ammonia gas produced reacts with HCl, it forms white fumes of ammonium chloride as,
$N{{H}_{3}}+HCl\to N{{H}_{4}}Cl$
Thus, we can say that,
A – ammonium nitrate
B – ammonia gas
White fumes produced – ammonium chloride
All this can be shown by a sample equation as,
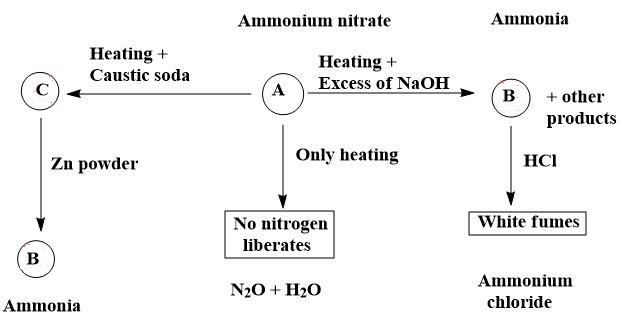
Therefore, option (C) is correct.
Note:
Do note to analyse each and every step to have a brief understanding of the whole equation and thus the reaction taking place.
As, we need to find only the composition of white fumes, we can solve only one side of the given equation. But for better understanding we should simplify each side of the reaction given (as finding ‘B’ would be simpler from the other path as $A\to C\to B$ )
Complete answer:
Let us move towards the given sample of equation to simplify it and find the required answer.
By analysing and having basic knowledge, let us say that the compound ‘A’ is ammonium nitrate i.e. $N{{H}_{4}}N{{O}_{3}}$ . Now, by moving step by step;
1. On thermal decomposition i.e. heating ammonium nitrate, it gives a mixture of ${{N}_{2}}O+{{H}_{2}}O$ as,
$N{{H}_{4}}N{{O}_{3}}\to {{N}_{2}}O+2{{H}_{2}}O$
2. On heating ammonium nitrate with caustic soda, it gives a mixture of $NaN{{O}_{3}}+N{{H}_{3}}+{{H}_{2}}O$ as,
$N{{H}_{4}}N{{O}_{3}}+NaOH\to NaN{{O}_{3}}+N{{H}_{3}}+{{H}_{2}}O$
Then the mixture of $NaN{{O}_{3}}+N{{H}_{3}}+{{H}_{2}}O$ on reduction with Zn powder gives a gas which is a replacement of ‘B’ i.e. ammonia gas.
3. On heating ammonium nitrate with excess NaOH, it gives ‘B’ i.e. ammonia gas along with other products. When the ammonia gas produced reacts with HCl, it forms white fumes of ammonium chloride as,
$N{{H}_{3}}+HCl\to N{{H}_{4}}Cl$
Thus, we can say that,
A – ammonium nitrate
B – ammonia gas
White fumes produced – ammonium chloride
All this can be shown by a sample equation as,

Therefore, option (C) is correct.
Note:
Do note to analyse each and every step to have a brief understanding of the whole equation and thus the reaction taking place.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




