
Which $A$ gives red colour in the reaction
\[A\xrightarrow[{(ii)NaOH}]{{(i)HN{O_2}}}red\;colour\]
1.$C{H_3}C{H_2}N{O_2}$
2.${(C{H_3})_2} - CHN{O_2}$
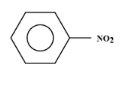
3.${(C{H_3})_3}N{O_2}$
4.

Answer
547.5k+ views
Hint:Nitro compounds have a nitro group attached to them which is \[\left( { - O - N = O} \right)\]. We can see that there are organic substances which have carbon atoms linked with a covalent bond to nitrogen atoms present in the nitro group. They are polar in nature and those with no other chemically reactive grouping are known to be colourless in nature.
Complete step-by-step answer:There are various nitro compounds which are produced commercially for usage as explosives, solvents or chemical intermediates. Nitration is the process for the formation of nitro compounds which occurs between nitric acid and an organic compound. For the nitration process of aromatic compounds such as benzene or toluene, they are present in a mixture of nitric acid and sulfuric acid at temperature \[100^\circ C\] or lower.
These temperatures are not high enough for nitrating aliphatic compounds such as propane which can b however commercially converted to a mixture of nitromethane, nitroethane by allowing it to react in the presence of nitric acid vapours at temperature of about \[400^\circ C\]. The mixture is then separated into its components by fractional distillation.
When nitromethane reacts with \[HN{O_2}\] first, the reaction results in the formation of nitrolic acid. This turned blue into color. Now when nitrolic acid reacts with $NaOH$, it turns a blue solution into a red color. Thus the option A which is $C{H_3}C{H_2}N{O_2}$ is the right answer.
Note:Reduction is the most important reaction among other reactions which occur with nitro compounds. Under acidic conditions, reduction almost always leads to the formation of an amine. In neutral media, reduction may result into the formation of a hydroxylamine. In an alkaline solution, compounds containing nitrogen-to-nitrogen bonds (azo, hydrazo, or azoxy compounds) are formed.
Complete step-by-step answer:There are various nitro compounds which are produced commercially for usage as explosives, solvents or chemical intermediates. Nitration is the process for the formation of nitro compounds which occurs between nitric acid and an organic compound. For the nitration process of aromatic compounds such as benzene or toluene, they are present in a mixture of nitric acid and sulfuric acid at temperature \[100^\circ C\] or lower.
These temperatures are not high enough for nitrating aliphatic compounds such as propane which can b however commercially converted to a mixture of nitromethane, nitroethane by allowing it to react in the presence of nitric acid vapours at temperature of about \[400^\circ C\]. The mixture is then separated into its components by fractional distillation.
When nitromethane reacts with \[HN{O_2}\] first, the reaction results in the formation of nitrolic acid. This turned blue into color. Now when nitrolic acid reacts with $NaOH$, it turns a blue solution into a red color. Thus the option A which is $C{H_3}C{H_2}N{O_2}$ is the right answer.
Note:Reduction is the most important reaction among other reactions which occur with nitro compounds. Under acidic conditions, reduction almost always leads to the formation of an amine. In neutral media, reduction may result into the formation of a hydroxylamine. In an alkaline solution, compounds containing nitrogen-to-nitrogen bonds (azo, hydrazo, or azoxy compounds) are formed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




