
Which will undergo the fastest $ {S_N}2 $ substitution reaction when treated with $ NaOH $ ?
(A)
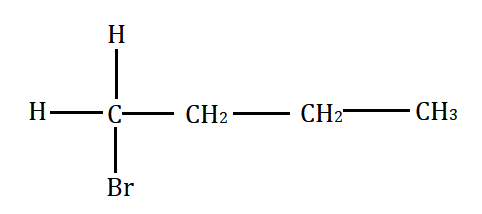
(B)
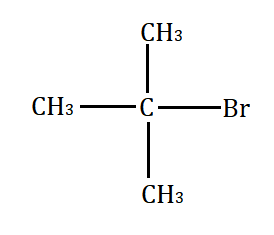
(C)
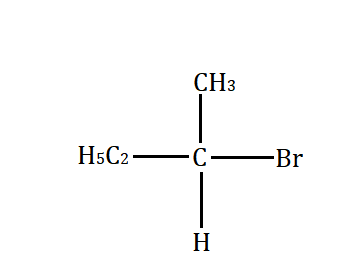
(D)
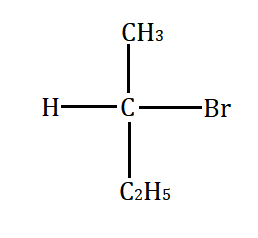
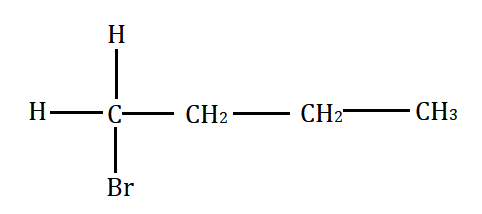



Answer
511.8k+ views
Hint :The $ {S_N}2 $ reaction is a nucleophilic substitution reaction where a bond is broken and another is formed synchronously. Two reacting species are involved in the rate determining step of the reaction. The term ' $ {S_N}2 $ ' stands for – Substitution Nucleophilic Bimolecular.
Complete Step By Step Answer:
The rate of reaction for $ {S_N}2 $ follows the following sequence if we take alkyl halides as substrate:
Primary alkyl halide $ > $ secondary alkyl halide $ > $ tertiary alkyl halide.
This happens because of the less steric hindrance for the attack by nucleophile to the primary alkyl halide. However, the steric hindrance increases from secondary to tertiary alkyl halide. Secondary alkyl halide prefers either $ {S_N}1 $ or $ {S_N}2 $ mechanism depending on the strength and solvent polarity. Tertiary alkyl halides prefer substitution through the $ {S_N}1 $ mechanism.
Keeping this in mind, the correct option for the above question is A.
Additional Information:
This reaction most often occurs at an aliphatic $ s{p^3} $ carbon centre with an electronegative, stable leaving group attached to it which is frequently a halide atom.
The $ {S_N}2 $ reaction type is very common and has other names, e.g., "bimolecular nucleophilic substitution", or, among inorganic chemists, "associative substitution" or "interchange mechanism".
Note :
Contrary to $ {S_N}2 $ , in the first step of $ {S_N}1 $ mechanism a carbonation is formed which is planer and hence attack of nucleophile (second step) may occur from either side to give a racemic product. However, there is no complete racemization as the nucleophilic species attacks the carbonation even before the departing halides ion has moved sufficiently away from the carbonation. In inorganic chemistry, the $ {S_N}1 $ reaction is often known as the dissociative mechanism.
Complete Step By Step Answer:
The rate of reaction for $ {S_N}2 $ follows the following sequence if we take alkyl halides as substrate:
Primary alkyl halide $ > $ secondary alkyl halide $ > $ tertiary alkyl halide.
This happens because of the less steric hindrance for the attack by nucleophile to the primary alkyl halide. However, the steric hindrance increases from secondary to tertiary alkyl halide. Secondary alkyl halide prefers either $ {S_N}1 $ or $ {S_N}2 $ mechanism depending on the strength and solvent polarity. Tertiary alkyl halides prefer substitution through the $ {S_N}1 $ mechanism.
Keeping this in mind, the correct option for the above question is A.
Additional Information:
This reaction most often occurs at an aliphatic $ s{p^3} $ carbon centre with an electronegative, stable leaving group attached to it which is frequently a halide atom.
The $ {S_N}2 $ reaction type is very common and has other names, e.g., "bimolecular nucleophilic substitution", or, among inorganic chemists, "associative substitution" or "interchange mechanism".
Note :
Contrary to $ {S_N}2 $ , in the first step of $ {S_N}1 $ mechanism a carbonation is formed which is planer and hence attack of nucleophile (second step) may occur from either side to give a racemic product. However, there is no complete racemization as the nucleophilic species attacks the carbonation even before the departing halides ion has moved sufficiently away from the carbonation. In inorganic chemistry, the $ {S_N}1 $ reaction is often known as the dissociative mechanism.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




