
Which type of overlapping is present in $S{O_3}$?
(A) $p\pi - p\pi $
(B) $p\pi - d\pi $
(C) $d\pi - d\pi $
(D) Both A and B
Answer
585.3k+ views
Hint: In the formation of bonds in $S{O_3}$ molecules, the d-orbitals of sulphur atoms take part. Oxygen atom forms a bond with a sulphur atom by its incompletely formed p-orbitals.
Complete step by step solution:
We know that according to Valence bond theory, it is required that overlapping between the atomic orbitals occurs in order to form a covalent bond.
-We know that a sigma ($\sigma $) bond forms when the overlap of an atom occurs between the nuclei of the respective atoms. A Pi ($\pi $) bond forms when the orbitals overlap outside the space between the respective nuclei. Now, let’s see the electronic configuration of both the atoms forming the molecule $S{O_3}$.
Electronic configuration of S :$1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}$
Electronic configuration of O :$1{s^2}2{s^2}2{p^4}$
Now, in order to form covalent bonds with three oxygen atoms, sulphur atoms undergo hybridization. The new electronic configuration of the excited sulphur atom will be:
Excited state electronic configuration of S: $1{s^2}2{s^2}2{p^6}3{s^1}3{p^3}3{d^2}$
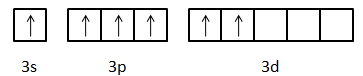
Now, we can see that there are six unpaired electrons are there in the valence orbit of the sulphur atom.
-One electron of s-orbital and two electrons of p-orbitals will form $\sigma $-bond with three oxygen atoms. Then the remaining one electron of p-orbital and two electrons of d-orbital will form $\pi $-bonds with those three oxygen atoms. So, we can draw the structure of $S{O_3}$ molecule as:
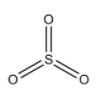
Here, we can see that oxygen will form bonds with its incompletely filled p-orbital. So, we can say that there will be overlapping between the $p\pi $ orbitals of sulphur atoms and $p\pi $ orbitals of oxygen atoms. So, there will be the presence of $p\pi - p\pi $ overlapping.
-There are unpaired electrons in the d-orbitals present in the excited state of the sulphur atom. So, there will be overlapping of $d\pi $ orbitals of sulphur and $p\pi $ orbitals of oxygen. So, we can also say that $d\pi - p\pi $ overlapping will be present in this molecule.
Therefore the correct answer is (D).
Note: Do not forget that orbitals of sulphur atoms undergo hybridization in the formation of $S{O_3}$. In the formation of $S{O_3}$, a total of three $\sigma $-bonds and three $\pi $-bonds are there.
Complete step by step solution:
We know that according to Valence bond theory, it is required that overlapping between the atomic orbitals occurs in order to form a covalent bond.
-We know that a sigma ($\sigma $) bond forms when the overlap of an atom occurs between the nuclei of the respective atoms. A Pi ($\pi $) bond forms when the orbitals overlap outside the space between the respective nuclei. Now, let’s see the electronic configuration of both the atoms forming the molecule $S{O_3}$.
Electronic configuration of S :$1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}$
Electronic configuration of O :$1{s^2}2{s^2}2{p^4}$
Now, in order to form covalent bonds with three oxygen atoms, sulphur atoms undergo hybridization. The new electronic configuration of the excited sulphur atom will be:
Excited state electronic configuration of S: $1{s^2}2{s^2}2{p^6}3{s^1}3{p^3}3{d^2}$

Now, we can see that there are six unpaired electrons are there in the valence orbit of the sulphur atom.
-One electron of s-orbital and two electrons of p-orbitals will form $\sigma $-bond with three oxygen atoms. Then the remaining one electron of p-orbital and two electrons of d-orbital will form $\pi $-bonds with those three oxygen atoms. So, we can draw the structure of $S{O_3}$ molecule as:

Here, we can see that oxygen will form bonds with its incompletely filled p-orbital. So, we can say that there will be overlapping between the $p\pi $ orbitals of sulphur atoms and $p\pi $ orbitals of oxygen atoms. So, there will be the presence of $p\pi - p\pi $ overlapping.
-There are unpaired electrons in the d-orbitals present in the excited state of the sulphur atom. So, there will be overlapping of $d\pi $ orbitals of sulphur and $p\pi $ orbitals of oxygen. So, we can also say that $d\pi - p\pi $ overlapping will be present in this molecule.
Therefore the correct answer is (D).
Note: Do not forget that orbitals of sulphur atoms undergo hybridization in the formation of $S{O_3}$. In the formation of $S{O_3}$, a total of three $\sigma $-bonds and three $\pi $-bonds are there.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




