
Which substance will conduct the current in the solid state?
A.Diamond
B.Graphite
C.Iodine
D.Sodium chloride
Answer
585.3k+ views
Hint: In solids, ions typically occupy fixed positions in the crystal lattice and do not move. However, ionic conduction can occur especially when the temperature increases.
Complete step by step answer:
Solids are basically incompressible which means that their particles are arranged very close to each other and there is no space between the constituent particles. Moreover, they are rigid and have definite mass and volume.
Basically, the electrical properties of solids are measured in terms of conductivity. All solids do not conduct electricity in equal amounts. So, on the basis of conduction of electricity, solids are classified as:
1.Conductors- These are the solids that can easily pass electric current through them. For example metals. Moreover, the electrical conductivity in metals is due to the presence of mobile electrons. There is no gap between conduction band and valence band and thus the electrons can easily flow from valence band to conduction band.
2.Semiconductors- In this case, the gap between conduction band and valence band is very less and thus the electron can jump from the valence band to conduction band. Moreover, their conductivity increases with an increase in temperature.
3.Insulators- These materials do not conduct electricity because the band gap between the conduction band and valence band is very large. Example wood, plastic etc.
Now, among the following options graphite can conduct electricity in the solid state. This is due to the fact that graphite has delocalized electrons just like metals. Moreover, these electrons are free to move between the layers in graphite and can conduct electricity. The forces between the layers are weak in graphite and this means that the layers can slide over each other. The structure of graphite is as shown:
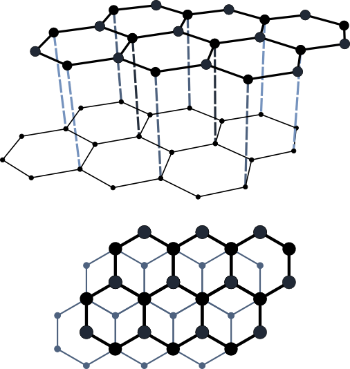
Hence, option B is correct.
Note: In case of diamond, each carbon atom is joined to four other carbon atoms and thus forming a giant covalent structure. It does not conduct electricity as there are no delocalized electrons in the structure whereas in case of iodine, its each molecule comprises two iodine atoms joined by a covalent bond and the electrical energy can’t be transferred and in case of sodium chloride also there are no free electrons present.
Complete step by step answer:
Solids are basically incompressible which means that their particles are arranged very close to each other and there is no space between the constituent particles. Moreover, they are rigid and have definite mass and volume.
Basically, the electrical properties of solids are measured in terms of conductivity. All solids do not conduct electricity in equal amounts. So, on the basis of conduction of electricity, solids are classified as:
1.Conductors- These are the solids that can easily pass electric current through them. For example metals. Moreover, the electrical conductivity in metals is due to the presence of mobile electrons. There is no gap between conduction band and valence band and thus the electrons can easily flow from valence band to conduction band.
2.Semiconductors- In this case, the gap between conduction band and valence band is very less and thus the electron can jump from the valence band to conduction band. Moreover, their conductivity increases with an increase in temperature.
3.Insulators- These materials do not conduct electricity because the band gap between the conduction band and valence band is very large. Example wood, plastic etc.
Now, among the following options graphite can conduct electricity in the solid state. This is due to the fact that graphite has delocalized electrons just like metals. Moreover, these electrons are free to move between the layers in graphite and can conduct electricity. The forces between the layers are weak in graphite and this means that the layers can slide over each other. The structure of graphite is as shown:

Hence, option B is correct.
Note: In case of diamond, each carbon atom is joined to four other carbon atoms and thus forming a giant covalent structure. It does not conduct electricity as there are no delocalized electrons in the structure whereas in case of iodine, its each molecule comprises two iodine atoms joined by a covalent bond and the electrical energy can’t be transferred and in case of sodium chloride also there are no free electrons present.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




