
Which statement(s) is/are WRONG?
A) Acetylene is insoluble in conc. \[{H_2}S{O_4}\] due to the non-formation of vinyl carbocation\[({H_3}C - \mathop C\limits^ \oplus {H_2})(HSO_4^ - )\]
B) Ethylene is soluble in conc. \[{H_2}S{O_4}\] due to the formation of vinyl carbocation \[({H_3}C - \mathop C\limits^ \oplus {H_2})(HSO_4^ - )\]
C) But-2-yne dissolves in conc. \[{H_2}S{O_4}\] due to the formation of vinyl carbocation \[(Me - \mathop C\limits^ \oplus = CH - Me)(HSO_4^ - )\]
D) More the s-character in the positively charged C, the more stable is the carbocation and more likely is its formation.
Answer
566.4k+ views
Hint: Solubility follows the rule “like dissolves like”. If a molecule is polar, it will dissolve in a polar solvent like concentrated \[{H_2}S{O_4}\]. That is, \[{H^ + }\]from \[{H_2}S{O_4}\]will attack the carbon atom to form carbocation only if there is some degree of polarity. Further, s-character increase means a lesser number of electron donating groups on the positively charged carbon.
Complete step-by-step answer:
Hydrocarbons (saturated or unsaturated) are insoluble in water as they are non-polar in nature. Water is a polar solvent and dissolves polar solutes. So, you can make unsaturated hydrocarbons water soluble, by protonation of them with strong acid such as sulphuric acid.
Statement A is correct because acetylene is a highly symmetrical and non-polar molecule and does not get affected by conc. \[{H_2}S{O_4}\]. In fact, conc. \[{H_2}S{O_4}\]is used as a drying agent in the preparation of acetylene.
Statement B is also correct because ethylene is a slightly polar molecule and \[{H^ + }\]from conc. \[{H_2}S{O_4}\]attacks one of the doubly bonded carbon-atoms to form a carbocation which forms a bond of ionic nature with and hence, is soluble in conc. \[{H_2}S{O_4}\].
Statement C is also a correct statement as methyl groups may be unsymmetrically distributed around doubly-bonded carbon which makes \[{H^ + }\] from conc. \[{H_2}S{O_4}\] to attack it and form a relatively stable carbocation whose combination with \[HSO_4^ - \] makes But-2-yne soluble in conc. \[{H_2}S{O_4}\]
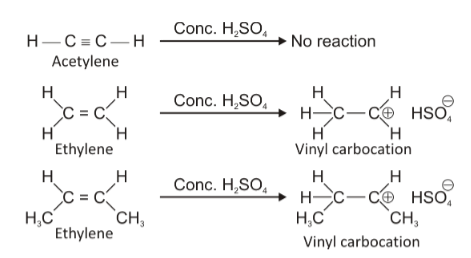
Statement D is a wrong statement because with increasing s-character, the stability of carbocation decreases. This happens because as the s-character increases, unsaturation increases, making electron donating alkyl groups less and less. Thus, the positive charge if acquired by such a carbon remains intensified on it and does not distribute or spread itself as happens in \[s{p^3}\]carbon. So, as the s-character increases, carbocation stability decreases because of intensification of positive charge.Thus option D is the wrong statement while all the above three statements are correct.
Hence, the correct answer is option ‘D’.
Note: London forces or weak van der Waals forces do not impact the dissolution process of a compound in conc. \[{H_2}S{O_4}\] as these forces are negligible as compared to strong polar forces that exist between \[{H_2}S{O_4}\]molecules. Further, the s-character increase, stabilizes the carbanion and not carbocation.
Complete step-by-step answer:
Hydrocarbons (saturated or unsaturated) are insoluble in water as they are non-polar in nature. Water is a polar solvent and dissolves polar solutes. So, you can make unsaturated hydrocarbons water soluble, by protonation of them with strong acid such as sulphuric acid.
Statement A is correct because acetylene is a highly symmetrical and non-polar molecule and does not get affected by conc. \[{H_2}S{O_4}\]. In fact, conc. \[{H_2}S{O_4}\]is used as a drying agent in the preparation of acetylene.
Statement B is also correct because ethylene is a slightly polar molecule and \[{H^ + }\]from conc. \[{H_2}S{O_4}\]attacks one of the doubly bonded carbon-atoms to form a carbocation which forms a bond of ionic nature with and hence, is soluble in conc. \[{H_2}S{O_4}\].
Statement C is also a correct statement as methyl groups may be unsymmetrically distributed around doubly-bonded carbon which makes \[{H^ + }\] from conc. \[{H_2}S{O_4}\] to attack it and form a relatively stable carbocation whose combination with \[HSO_4^ - \] makes But-2-yne soluble in conc. \[{H_2}S{O_4}\]

Statement D is a wrong statement because with increasing s-character, the stability of carbocation decreases. This happens because as the s-character increases, unsaturation increases, making electron donating alkyl groups less and less. Thus, the positive charge if acquired by such a carbon remains intensified on it and does not distribute or spread itself as happens in \[s{p^3}\]carbon. So, as the s-character increases, carbocation stability decreases because of intensification of positive charge.Thus option D is the wrong statement while all the above three statements are correct.
Hence, the correct answer is option ‘D’.
Note: London forces or weak van der Waals forces do not impact the dissolution process of a compound in conc. \[{H_2}S{O_4}\] as these forces are negligible as compared to strong polar forces that exist between \[{H_2}S{O_4}\]molecules. Further, the s-character increase, stabilizes the carbanion and not carbocation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




