
Which statement is correct about the following reaction?
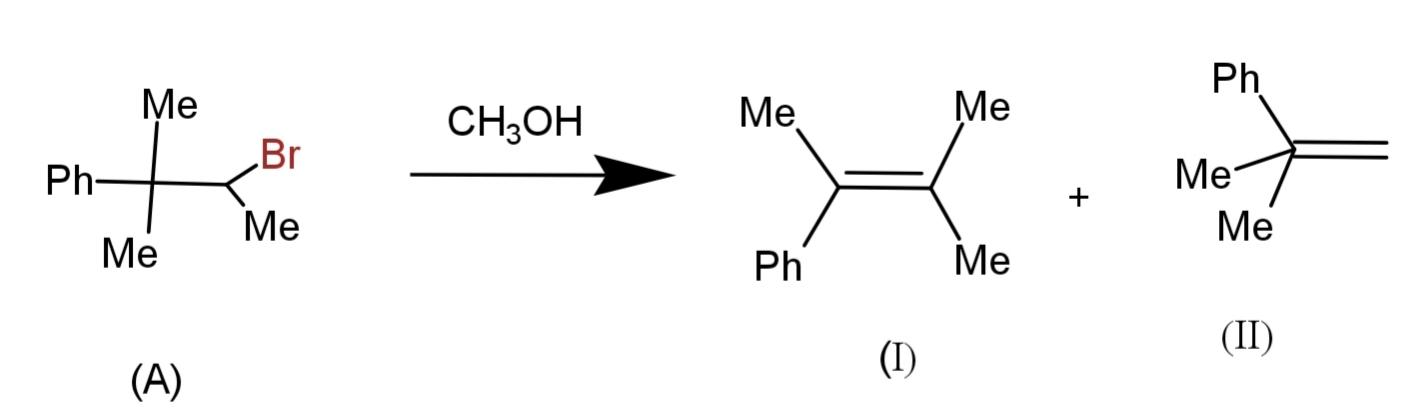
A. The major product is (I) by E1 mechanism
B. The major product is (I) by E2 mechanism
C. The major product is (II) by E1 mechanism
D. The major product is (II) by E2 mechanism

Answer
512.7k+ views
Hint: the reactions used to convert saturated molecules into unsaturated ones are called elimination reactions. Elimination reactions include the removal of the substituent attached with the saturated molecule. These reactions are of 2 types E1 and E2. E1 reaction is uni -molecular, while the E2 reaction is bi – molecular.
Complete answer:
We have been given an elimination reaction. We have to determine which of the products is the major and the mechanism that is either E1 elimination or E2 elimination.
Elimination reactions are used to convert saturated compounds to unsaturated compounds by the use of a catalyst of alcohol or any other acid. The elimination is of two types E1 and E2. They differ in being uni and bi molecular reactions. E1 mechanism is similar to ${{S}_{N}}^{1}$ reaction and happens in two steps and E2 is similar to ${{S}_{N}}^{2}$ reaction and happens in a single step.
The given reaction consists of (A) as a reactant that is a secondary alkyl halide. The mechanism involves ionization and deprotonation as:
The steps being involved are two as the reactant is bulky and elimination will happen in two steps, therefore it is E1 elimination. The major product is (I) because the secondary carbocation is more stable and will form the major product, while (II) is formed by the primary carbocation.
Hence, the major product is (I) by E1 mechanism.
So option A is correct.
Note:
The E1 elimination is suitable for bulky alkyl halides (tertiary) as they cannot be converted in one step by E2 elimination. Also the order of reaction of carbocation for E1 is similar to ${{S}_{N}}^{1}$ that is ${{3}^{o}}>{{2}^{o}}>{{1}^{o}}$. The E2 elimination requires a strong base, but E1 elimination can occur in presence of a weak base.
Complete answer:
We have been given an elimination reaction. We have to determine which of the products is the major and the mechanism that is either E1 elimination or E2 elimination.
Elimination reactions are used to convert saturated compounds to unsaturated compounds by the use of a catalyst of alcohol or any other acid. The elimination is of two types E1 and E2. They differ in being uni and bi molecular reactions. E1 mechanism is similar to ${{S}_{N}}^{1}$ reaction and happens in two steps and E2 is similar to ${{S}_{N}}^{2}$ reaction and happens in a single step.
The given reaction consists of (A) as a reactant that is a secondary alkyl halide. The mechanism involves ionization and deprotonation as:
The steps being involved are two as the reactant is bulky and elimination will happen in two steps, therefore it is E1 elimination. The major product is (I) because the secondary carbocation is more stable and will form the major product, while (II) is formed by the primary carbocation.
Hence, the major product is (I) by E1 mechanism.
So option A is correct.
Note:
The E1 elimination is suitable for bulky alkyl halides (tertiary) as they cannot be converted in one step by E2 elimination. Also the order of reaction of carbocation for E1 is similar to ${{S}_{N}}^{1}$ that is ${{3}^{o}}>{{2}^{o}}>{{1}^{o}}$. The E2 elimination requires a strong base, but E1 elimination can occur in presence of a weak base.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




