
Which statement is correct about cyclopentadienyl anion?
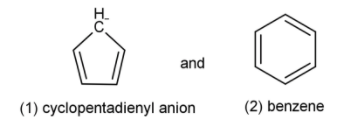
A) Both (1) and (2) are aromatic but (2) is more stable than (1).
B) Both (1) and (2) are aromatic and have the same stability
C) (2) is more aromatic and more stable than (1) and it is non-aromatic.
D) (1) is more stable than (2) though both are aromatic.

Answer
597k+ views
Hint: A compound is said to be aromatic if it has (4n+2)π electrons which are delocalised in the structure and the structure is planar. Also remember that neutral structures are always more stable than charged structures.
Complete step by step answer:
First we should start from the aromaticity of these 2 structures. To be known as an aromatic compound any structure should fulfil following conditions:
1. The molecule should be cyclic
2. These π electrons should be delocalised in the ring or structure
3. The structure should be planar
4. It should follow Hückel’s rule or the (4n+2) rule.
According to the Hückel’s rule (also known as (4n+2) rule) any compound having number of π electrons equal to (4n+2) where n is 0 or any other whole natural number (1, 2, 3 ….) is said to be an aromatic compound.
For benzene: it has 6π electrons which is equal to (4n+2)π electrons, where n = 1. So, we can say that benzene follows Hückel's rule.
For cyclopentadienyl anion: it also has 6π electrons which is equal to (4n+2)π electrons, where n = 1. So, we can say that this also follows Hückel's rule.
So let us see if cyclopentadienyl anion and benzene structure are aromatic or not:
Cyclopentadienyl anion (${\left[ {{C_5}{H_5}} \right]^{ - 1}}_{}$): It has 6π electrons (2 at position of negative charge and 4 from the two double bonds) thus following the Hückel’s rule, these electrons are delocalised in the entire ring forming various resonance structures and also the structure is a planar structure. So, it is aromatic.
Benzene (${C_6}{H_6}$): It has 6π electrons (2 from each of the 3 double bonds, so in total 6π electrons) thus following the Hückel’s rule, these electrons are delocalised on the entire ring and forms resonating structures and also the benzene ring is planar. So, it also is aromatic.
Hence we can conclude that both cyclopentadienyl anion and benzene are aromatic.
Now let us come to the point of stability. We already know that neutral structures are more stable than charged structures.
So, we can say that benzene is more stable than cyclopentadienyl anion. Another reason for stability of benzene is less angle strain in its structure.
Hence from the above discussion we have come to a conclusion that: cyclopentadienyl anion and benzene both are aromatic and benzene is more stable among both of them.
So, the correct option is: A) Both (1) and (2) are aromatic but (2) is more stable than (1).
Note: Major mistake that we can make here is while finding a compound’s aromaticity. Always make sure you declare any compound aromatic only if it fulfils all required conditions. Even if it fails to fulfil one single condition it cannot be called aromatic.
Complete step by step answer:
First we should start from the aromaticity of these 2 structures. To be known as an aromatic compound any structure should fulfil following conditions:
1. The molecule should be cyclic
2. These π electrons should be delocalised in the ring or structure
3. The structure should be planar
4. It should follow Hückel’s rule or the (4n+2) rule.
According to the Hückel’s rule (also known as (4n+2) rule) any compound having number of π electrons equal to (4n+2) where n is 0 or any other whole natural number (1, 2, 3 ….) is said to be an aromatic compound.
For benzene: it has 6π electrons which is equal to (4n+2)π electrons, where n = 1. So, we can say that benzene follows Hückel's rule.
For cyclopentadienyl anion: it also has 6π electrons which is equal to (4n+2)π electrons, where n = 1. So, we can say that this also follows Hückel's rule.
So let us see if cyclopentadienyl anion and benzene structure are aromatic or not:
Cyclopentadienyl anion (${\left[ {{C_5}{H_5}} \right]^{ - 1}}_{}$): It has 6π electrons (2 at position of negative charge and 4 from the two double bonds) thus following the Hückel’s rule, these electrons are delocalised in the entire ring forming various resonance structures and also the structure is a planar structure. So, it is aromatic.
Benzene (${C_6}{H_6}$): It has 6π electrons (2 from each of the 3 double bonds, so in total 6π electrons) thus following the Hückel’s rule, these electrons are delocalised on the entire ring and forms resonating structures and also the benzene ring is planar. So, it also is aromatic.
Hence we can conclude that both cyclopentadienyl anion and benzene are aromatic.
Now let us come to the point of stability. We already know that neutral structures are more stable than charged structures.
So, we can say that benzene is more stable than cyclopentadienyl anion. Another reason for stability of benzene is less angle strain in its structure.
Hence from the above discussion we have come to a conclusion that: cyclopentadienyl anion and benzene both are aromatic and benzene is more stable among both of them.
So, the correct option is: A) Both (1) and (2) are aromatic but (2) is more stable than (1).
Note: Major mistake that we can make here is while finding a compound’s aromaticity. Always make sure you declare any compound aromatic only if it fulfils all required conditions. Even if it fails to fulfil one single condition it cannot be called aromatic.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




