
Which species has the same shape as the $N{{O}_{3}}^{-}$?
A. $S{{O}_{3}}$
B. $S{{O}_{3}}^{2-}$
C. $Cl{{F}_{3}}^{-}$
D. $Cl{{O}_{3}}^{-}$
Answer
576k+ views
Hint: The atoms which are isoelectronic in nature will share the same properties i.e. they will have the same number of electrons, same shape and other properties.
Complete step by step solution:
As per the given question we have to find out which species will share the same shape with $N{{O}_{3}}^{-}$. So, as we know in chemistry the species having the same number of electrons (i.e. if they are isoelectronic in nature) in their structure will share the same properties which mean both the species will have the same shape, atomic size, bonds, and other properties. So, Here, the number of electrons $N{{O}_{3}}^{-}$ will have is $(7+3\times 8)+1=32 electrons$ in total. Likewise, we have to find out the number of electrons of the given species so as to see which species is isoelectronic with $N{{O}_{3}}^{-}$.
Thus, The number of electrons $S{{O}_{3}}$ will have is 32.
The number of electrons $S{{O}_{3}}^{2-}$ will have is 34.
The number of electrons $Cl{{F}_{3}}^{-}$ will have is 45.
The number of electrons $Cl{{O}_{3}}^{-}$ will have 42.
So, we can see that $S{{O}_{3}}$is having the same electrons as the $N{{O}_{3}}^{-}$. Thus, $S{{O}_{3}}$ will have the same shape as the $N{{O}_{3}}^{-}$. In nitrate ion i.e. $N{{O}_{3}}^{-}$, where it share a $s{{p}^{2}}$ type of hybridisation and there is one central atom i.e. nitrogen which is surrounded by three identically bonded oxygen atoms that lie at the corners of a triangle and at the same one-dimensional plane. It has no lone pairs and has three electron domains. Hence, nitrate ion molecular geometry is slightly bent giving a trigonal planar structure.
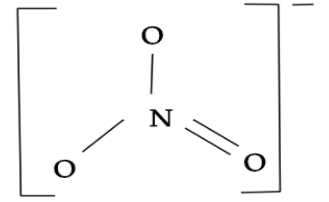
While, sulphur trioxide i.e. $S{{O}_{3}}$ also shares a $s{{p}^{2}}$ type of hybridisation and here sulphur will be the central atom which is surrounded by three identically bonded oxygen atoms. It has no lone pairs and the molecular geometry it has is trigonal planar.
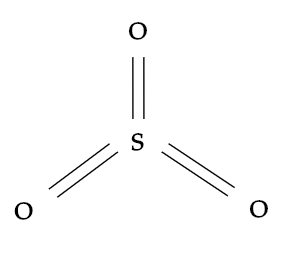
Hence, the correct option is A.
Note: Species sharing the same hybridisation and number of electrons will have the same molecular geometry. As well as, those species who are isoelectronic in nature will have the same properties like atomic radii, melting point, boiling point, etc.
Complete step by step solution:
As per the given question we have to find out which species will share the same shape with $N{{O}_{3}}^{-}$. So, as we know in chemistry the species having the same number of electrons (i.e. if they are isoelectronic in nature) in their structure will share the same properties which mean both the species will have the same shape, atomic size, bonds, and other properties. So, Here, the number of electrons $N{{O}_{3}}^{-}$ will have is $(7+3\times 8)+1=32 electrons$ in total. Likewise, we have to find out the number of electrons of the given species so as to see which species is isoelectronic with $N{{O}_{3}}^{-}$.
Thus, The number of electrons $S{{O}_{3}}$ will have is 32.
The number of electrons $S{{O}_{3}}^{2-}$ will have is 34.
The number of electrons $Cl{{F}_{3}}^{-}$ will have is 45.
The number of electrons $Cl{{O}_{3}}^{-}$ will have 42.
So, we can see that $S{{O}_{3}}$is having the same electrons as the $N{{O}_{3}}^{-}$. Thus, $S{{O}_{3}}$ will have the same shape as the $N{{O}_{3}}^{-}$. In nitrate ion i.e. $N{{O}_{3}}^{-}$, where it share a $s{{p}^{2}}$ type of hybridisation and there is one central atom i.e. nitrogen which is surrounded by three identically bonded oxygen atoms that lie at the corners of a triangle and at the same one-dimensional plane. It has no lone pairs and has three electron domains. Hence, nitrate ion molecular geometry is slightly bent giving a trigonal planar structure.

While, sulphur trioxide i.e. $S{{O}_{3}}$ also shares a $s{{p}^{2}}$ type of hybridisation and here sulphur will be the central atom which is surrounded by three identically bonded oxygen atoms. It has no lone pairs and the molecular geometry it has is trigonal planar.

Hence, the correct option is A.
Note: Species sharing the same hybridisation and number of electrons will have the same molecular geometry. As well as, those species who are isoelectronic in nature will have the same properties like atomic radii, melting point, boiling point, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




