
Which row of the table is correct? Increasing number of carbon atoms —
A) Ethyl methanote Methyl propanoate Pentyl pentanoate Propyl butanoate B) ethyl methanoate methyl propanoate propyl butanoate pentyl pentanoate C) methyl propanoate propyl butanoate ethyl methanoate pentyl pentanoate D) propyl butanoate ethyl methanoate pentyl pentanoate methyl propanoate
A) A
B) B
C) C
D) D
| A) | Ethyl methanote | Methyl propanoate | Pentyl pentanoate | Propyl butanoate |
| B) | ethyl methanoate | methyl propanoate | propyl butanoate | pentyl pentanoate |
| C) | methyl propanoate | propyl butanoate | ethyl methanoate | pentyl pentanoate |
| D) | propyl butanoate | ethyl methanoate | pentyl pentanoate | methyl propanoate |
Answer
565.2k+ views
Hint: Esters are organic compounds. These compounds have a functional group $\text{ }-\text{COOR}$ . It is derived from the acid where $\text{ }-\text{OH }$that is the hydroxyl group is replaced by the alkoxy $\text{ }-\text{OR}$ group. The general representation of ester is as follows,
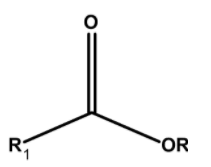
The IUPAC nomenclature involves the two parts. The compound is visually separated from the oxygen atom adjacent to the carbonyl group. The group which is from the acid part is ended by the –oate.
Complete answer:
The ester is the organic compound. To determine the name of a compound. Follows these steps:
1) First identify the carbon which is the part of the continuous chin which is bonded to the carbon on both sides (one from alkyl and the other from carboxyl).
2) Then start the numbering by considering the oxygen on either side.
3) The general format is,
(Alkyl on the side further from the carbonyl) (Alkane on the other side of carbonyl)
4) Replace the ending of alkane .change the –e to the -oate.
Here we are interested to determine the increasing number of carbon atoms in the compounds.
The structure of ethyl methanoate has ethyl groups $\text{ }-{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{ }$and $\text{ }-\text{COC}{{\text{H}}_{\text{3}}}\text{ }$ on either side of the oxygen. Thus the structure of ethyl methanoate is as follows,
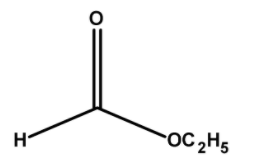
Thus, the ester ethyl methanoate has a total $\text{ 2 + 1 = 3 }$ number of carbon atoms.
The structure of methyl propanoate has ethyl groups $\text{ }-\text{C}{{\text{H}}_{3}}\text{ }$and $\text{ }-\text{COC}{{\text{H}}_{2}}\text{C}{{\text{H}}_{\text{3}}}\text{ }$ on either side of the oxygen. Thus the structure of ethyl methanoate is as follows,
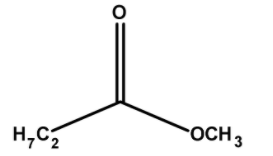
Thus, the ester methyl propanoate has a total $\text{ 1 + 3 = 4 }$ number of carbon atoms.
The structure of propyl butanoate have propyl groups $\text{ }-\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{3}}}\text{ }$and $\text{ }-\text{COC}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{\text{3}}}\text{ }$ on either side of the oxygen. Thus the structure of ethyl methanoate is as follows,
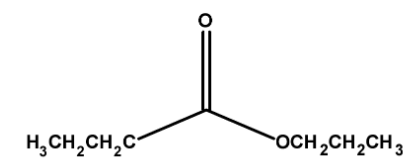
Thus, the ester methyl propanoate has a total $\text{ 3 + 4 = 7 }$ number of carbon atoms.
The structure of pentyl pentanoate has pentyl groups $\text{ }-\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{3}}}\text{ }$and $\text{ }-\text{COC}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{\text{3}}}\text{ }$ on either side of the oxygen. Thus the structure of ethyl methanoate is as follows,
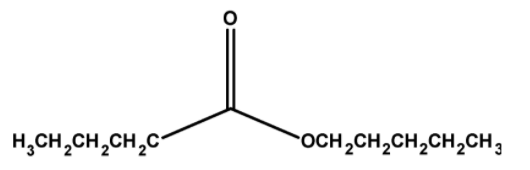
Thus, the ester methyl propanoate has a total $\text{ 5 + 5 = 10 }$ number of carbon atoms.
Thus correct order of increasing number of carbon atom is,
$\text{ }\begin{matrix}
\text{Ethyl methanoate }<\text{ Methyl propanoate }<\text{ propyl butanoate }<\text{ pentyl pentanoate} \\
(\text{Increasing number of carbon atom }\to \text{)} \\
\end{matrix}$
Hence, (B) is the correct option.
Note:
Note that esters are derived from the carboxylic acid. In naming the compound, visually we can see alcohol and the acid in the ester. The part which has the acid groups is named as the acid (-ic) and the –ic is replaced by the suffix –ate. The alkyl groups of the alcohol part are written in front of the acid part.to draw the structure, first, write down the $\text{ }-\text{O}-\text{C}=\text{O}$ groups and attached the alkyl part on the oxygen atom and –ate ending carbon atoms on the carboxyl carbon atoms.

The IUPAC nomenclature involves the two parts. The compound is visually separated from the oxygen atom adjacent to the carbonyl group. The group which is from the acid part is ended by the –oate.
Complete answer:
The ester is the organic compound. To determine the name of a compound. Follows these steps:
1) First identify the carbon which is the part of the continuous chin which is bonded to the carbon on both sides (one from alkyl and the other from carboxyl).
2) Then start the numbering by considering the oxygen on either side.
3) The general format is,
(Alkyl on the side further from the carbonyl) (Alkane on the other side of carbonyl)
4) Replace the ending of alkane .change the –e to the -oate.
Here we are interested to determine the increasing number of carbon atoms in the compounds.
The structure of ethyl methanoate has ethyl groups $\text{ }-{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{ }$and $\text{ }-\text{COC}{{\text{H}}_{\text{3}}}\text{ }$ on either side of the oxygen. Thus the structure of ethyl methanoate is as follows,

Thus, the ester ethyl methanoate has a total $\text{ 2 + 1 = 3 }$ number of carbon atoms.
The structure of methyl propanoate has ethyl groups $\text{ }-\text{C}{{\text{H}}_{3}}\text{ }$and $\text{ }-\text{COC}{{\text{H}}_{2}}\text{C}{{\text{H}}_{\text{3}}}\text{ }$ on either side of the oxygen. Thus the structure of ethyl methanoate is as follows,

Thus, the ester methyl propanoate has a total $\text{ 1 + 3 = 4 }$ number of carbon atoms.
The structure of propyl butanoate have propyl groups $\text{ }-\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{3}}}\text{ }$and $\text{ }-\text{COC}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{\text{3}}}\text{ }$ on either side of the oxygen. Thus the structure of ethyl methanoate is as follows,

Thus, the ester methyl propanoate has a total $\text{ 3 + 4 = 7 }$ number of carbon atoms.
The structure of pentyl pentanoate has pentyl groups $\text{ }-\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{3}}}\text{ }$and $\text{ }-\text{COC}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{2}}\text{C}{{\text{H}}_{\text{3}}}\text{ }$ on either side of the oxygen. Thus the structure of ethyl methanoate is as follows,

Thus, the ester methyl propanoate has a total $\text{ 5 + 5 = 10 }$ number of carbon atoms.
Thus correct order of increasing number of carbon atom is,
$\text{ }\begin{matrix}
\text{Ethyl methanoate }<\text{ Methyl propanoate }<\text{ propyl butanoate }<\text{ pentyl pentanoate} \\
(\text{Increasing number of carbon atom }\to \text{)} \\
\end{matrix}$
Hence, (B) is the correct option.
Note:
Note that esters are derived from the carboxylic acid. In naming the compound, visually we can see alcohol and the acid in the ester. The part which has the acid groups is named as the acid (-ic) and the –ic is replaced by the suffix –ate. The alkyl groups of the alcohol part are written in front of the acid part.to draw the structure, first, write down the $\text{ }-\text{O}-\text{C}=\text{O}$ groups and attached the alkyl part on the oxygen atom and –ate ending carbon atoms on the carboxyl carbon atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




