
Which products would actually form in the reaction shown above?
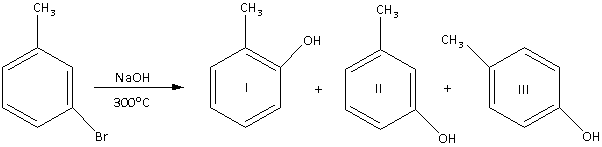
A. I
B. III
C. II
D. I and II

Answer
584.7k+ views
Hint: m-bromotoluene reacts with ${\text{NaOH}}$ at an elevated temperature of ${300^ \circ }{\text{C}}$. In the reaction, the nucleophile \[{\text{O}}{{\text{H}}^ - }\] substitutes the ${\text{Br}}$ atom attached to an aromatic ring. Thus, the reaction is an aromatic nucleophilic substitution reaction.
${\text{NaOH}}$ is a very strong base. Thus, the reaction occurs in a strongly basic medium. Thus, the reaction occurs through formation of benzyne as an intermediate.
Complete step by step answer:
In the reaction, m-bromotoluene reacts with ${\text{NaOH}}$ at an elevated temperature of ${300^ \circ }{\text{C}}$.
In the reaction, the nucleophile \[{\text{O}}{{\text{H}}^ - }\] substitutes the ${\text{Br}}$ atom attached to an aromatic ring. Thus, the reaction is an aromatic nucleophilic substitution reaction.
${\text{NaOH}}$ is a very strong base. Thus, the reaction occurs in a strongly basic medium. Thus, the reaction occurs through formation of benzyne as an intermediate.
The mechanism of the reaction is as follows:
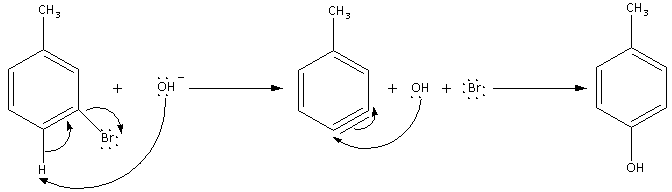
Thus, the product formed in the reaction of m-bromotoluene is 4-hydroxytoluene.
So, the correct answer is “Option B”.
Additional Information: Benzyne is a derivative of an aromatic ring. Benzyne is formed by removing two substituents from the aromatic ring. Benzyne contains non-linear triple bonds. Thus, benzyne is highly strained. and is highly reactive.
Benzyne is very unstable in nature and can be attacked by nucleophiles at any end.
Note: In the reaction, strong base ${\text{NaOH}}$ is used. Thus, the reaction occurs in a strongly basic medium. Strong basic medium indicates that the reaction will proceed via formation of benzyne intermediate.
Benzyne is a derivative of an aromatic ring formed by removal of two substituents. The structure of benzyne is similar to that of benzene with on additional $\pi $-bond.
${\text{NaOH}}$ is a very strong base. Thus, the reaction occurs in a strongly basic medium. Thus, the reaction occurs through formation of benzyne as an intermediate.
Complete step by step answer:
In the reaction, m-bromotoluene reacts with ${\text{NaOH}}$ at an elevated temperature of ${300^ \circ }{\text{C}}$.
In the reaction, the nucleophile \[{\text{O}}{{\text{H}}^ - }\] substitutes the ${\text{Br}}$ atom attached to an aromatic ring. Thus, the reaction is an aromatic nucleophilic substitution reaction.
${\text{NaOH}}$ is a very strong base. Thus, the reaction occurs in a strongly basic medium. Thus, the reaction occurs through formation of benzyne as an intermediate.
The mechanism of the reaction is as follows:

Thus, the product formed in the reaction of m-bromotoluene is 4-hydroxytoluene.
So, the correct answer is “Option B”.
Additional Information: Benzyne is a derivative of an aromatic ring. Benzyne is formed by removing two substituents from the aromatic ring. Benzyne contains non-linear triple bonds. Thus, benzyne is highly strained. and is highly reactive.
Benzyne is very unstable in nature and can be attacked by nucleophiles at any end.
Note: In the reaction, strong base ${\text{NaOH}}$ is used. Thus, the reaction occurs in a strongly basic medium. Strong basic medium indicates that the reaction will proceed via formation of benzyne intermediate.
Benzyne is a derivative of an aromatic ring formed by removal of two substituents. The structure of benzyne is similar to that of benzene with on additional $\pi $-bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




