
Which product is formed when diethyl ether is exposed to sunlight and air for a long period?
A.Peroxide
B.Ethyl alcohol
C.Diethyl ketone
D.Ethane
Answer
568.2k+ views
Hint: Ether is a compound where two alkyls or aryl or one alkyl and one aryl group is bonded with oxygen. It can be formed by the dehydration of alcohols by emitting water molecules using dehydrating agents like Conc. $H_2SO_4$
Complete step by step answer:
Ether is a volatile, flammable, colorless liquid with a distinctive odor. It belongs to the large functional group of organic compounds called ethers. Its IUPAC name is alkoxy alkane. Ether is synthesized by dehydration (removal of a water molecule) of alcohol using sulfuric acid. For example, the reaction of synthesis of diethyl ether shown below,
\[2C{H_3}C{H_2}OH + 2{H_2}S{O_4} \to {(C{H_3}C{H_2})_2}O + {H_2}S{O_4} + {H_2}O\]
Ether undergoes combustion reaction, reacts with oxygen, and forms carbon dioxide and water. It is highly flammable and reacts with halogens like chlorine or bromine to form halogen-substituted ether that undergoes substitution reaction in the absence of sunlight.
In presence of sunlight ethers form peroxides. The reaction is shown below,
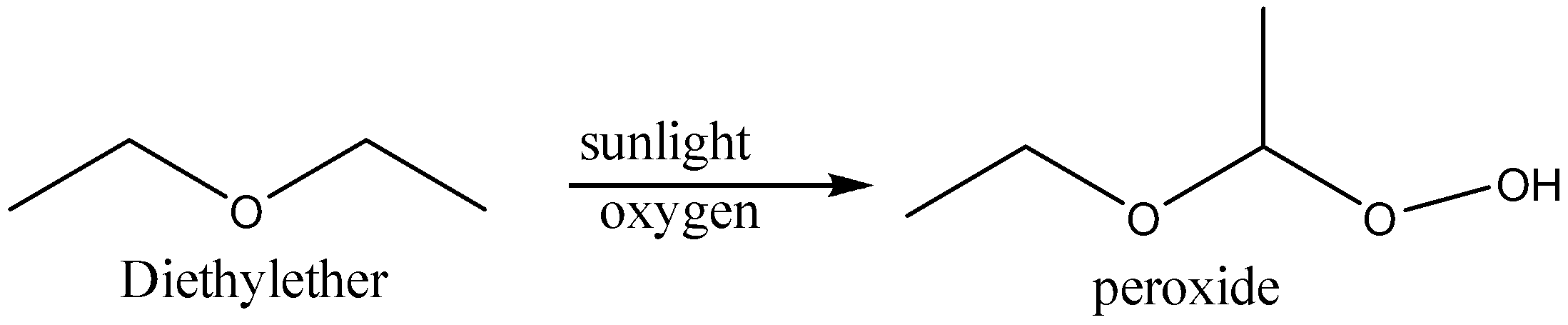
So, the correct option is A.
Additional information:
In presence of an acid, ether can be dissociated. For example, in the presence of HI, it dissociates by the following mechanism.
\[RO{R'}\xrightarrow{{HI}}ROH + {R'}I\xrightarrow{{HI}}RI + {R'}I\]
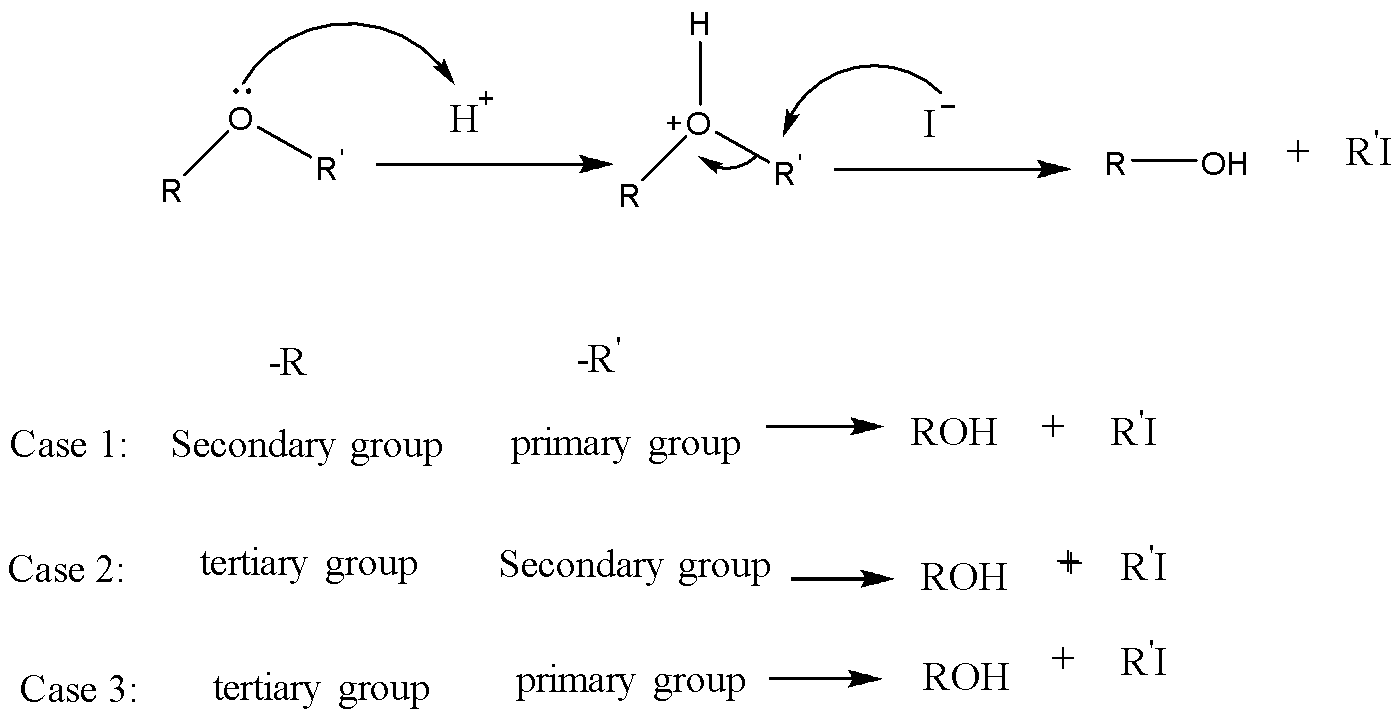
This mechanism is the\[S{N^2}\] mechanism. The formation of alkyl iodide and alcohol depends upon the group attached to the oxygen. Between secondary and Primary alkyl groups, the primary alkyl group favors the \[S{N^2}\] attack at the iodide nucleophile to form corresponding alkyl iodide. Between secondary and tertiary alkyl groups, the secondary alkyl group favors the \[S{N^2}\] attack at the iodide nucleophile to form corresponding alkyl iodide. And between tertiary and Primary alkyl groups, the primary alkyl group favors the \[S{N^2}\] attack at the iodide nucleophile to form corresponding alkyl iodide. In between the vinyl or benzyl group and an alkyl group, the alkyl group favors the \[S{N^2}\] attack at the iodide nucleophile to form corresponding alkyl iodide. Because vinyl and benzyl groups do not participate in the\[S{N^2}\] reaction.
Now for the phenyl methyl ether in presence of HI, it will dissociate in the following manner,
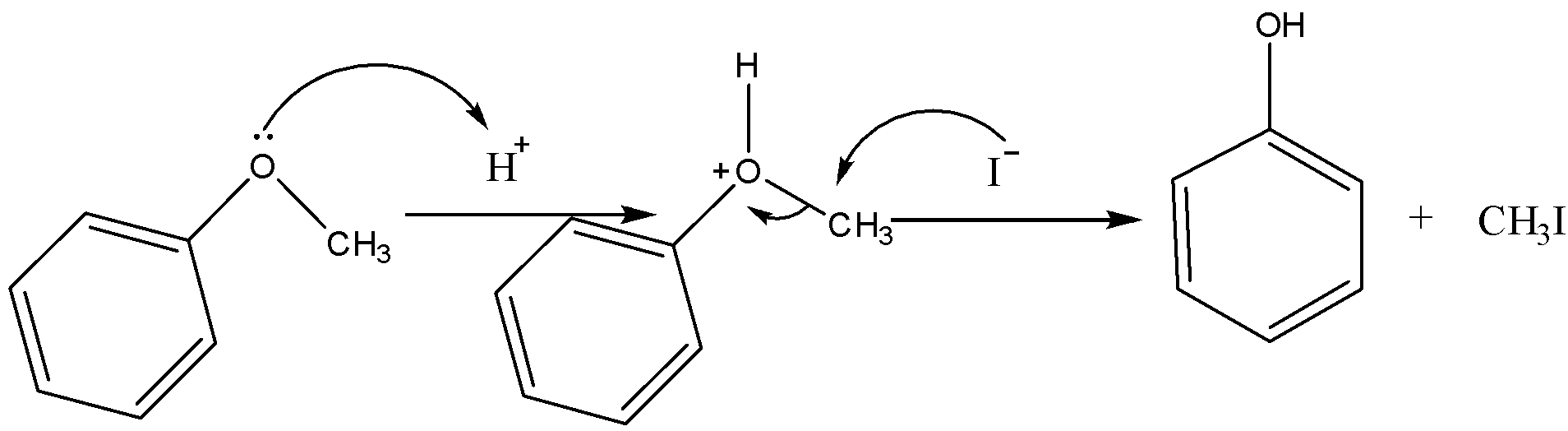
Therefore, on the heating of phenyl methyl ether in presence of phenol is formed with methyl iodide.
Note: From the structure, we get that ethers do not have a hydrogen-bonding network that needs to be broken up to dissolve the solute. It is a nonpolar molecule similar in structure to alcohol, and both ethers and alcohols are similar in structure to water.
Complete step by step answer:
Ether is a volatile, flammable, colorless liquid with a distinctive odor. It belongs to the large functional group of organic compounds called ethers. Its IUPAC name is alkoxy alkane. Ether is synthesized by dehydration (removal of a water molecule) of alcohol using sulfuric acid. For example, the reaction of synthesis of diethyl ether shown below,
\[2C{H_3}C{H_2}OH + 2{H_2}S{O_4} \to {(C{H_3}C{H_2})_2}O + {H_2}S{O_4} + {H_2}O\]
Ether undergoes combustion reaction, reacts with oxygen, and forms carbon dioxide and water. It is highly flammable and reacts with halogens like chlorine or bromine to form halogen-substituted ether that undergoes substitution reaction in the absence of sunlight.
In presence of sunlight ethers form peroxides. The reaction is shown below,

So, the correct option is A.
Additional information:
In presence of an acid, ether can be dissociated. For example, in the presence of HI, it dissociates by the following mechanism.
\[RO{R'}\xrightarrow{{HI}}ROH + {R'}I\xrightarrow{{HI}}RI + {R'}I\]

This mechanism is the\[S{N^2}\] mechanism. The formation of alkyl iodide and alcohol depends upon the group attached to the oxygen. Between secondary and Primary alkyl groups, the primary alkyl group favors the \[S{N^2}\] attack at the iodide nucleophile to form corresponding alkyl iodide. Between secondary and tertiary alkyl groups, the secondary alkyl group favors the \[S{N^2}\] attack at the iodide nucleophile to form corresponding alkyl iodide. And between tertiary and Primary alkyl groups, the primary alkyl group favors the \[S{N^2}\] attack at the iodide nucleophile to form corresponding alkyl iodide. In between the vinyl or benzyl group and an alkyl group, the alkyl group favors the \[S{N^2}\] attack at the iodide nucleophile to form corresponding alkyl iodide. Because vinyl and benzyl groups do not participate in the\[S{N^2}\] reaction.
Now for the phenyl methyl ether in presence of HI, it will dissociate in the following manner,

Therefore, on the heating of phenyl methyl ether in presence of phenol is formed with methyl iodide.
Note: From the structure, we get that ethers do not have a hydrogen-bonding network that needs to be broken up to dissolve the solute. It is a nonpolar molecule similar in structure to alcohol, and both ethers and alcohols are similar in structure to water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




