
Which oxyacid of sulphur contains S-S single bond?
A.Oleum
B.Marshall’s acid
C.Dithionic acid
D.Thiosulphuric acid
Answer
582.6k+ views
Hint: In oxyacid of sulphur, sulphur is the central atom exhibiting a tetrahedral structure when coordinated with oxygen. This contains at least one \[S = O\] bond and one \[S - OH\] bond. In addition to these, a chain of \[{( - S - )_n}\] as in \[{H_2}{S_2}{O_6}\] . Such oxyacids with \[S - S\] linkages are called Thioacids.
Complete step-by-step solution:
The sulphur oxyacids are the acids that contain oxygen, hydrogen and sulphur. These are divides into four groups based on their structural similarities:
Sulphurous acid group- these are prepared by dissolving sulphur dioxide in water. Their structure is pyramidal with three oxygen atoms on a triangle i.e. tetrahedral structure is distorted by lone pairs. These are strong reducing agents and have bleaching properties.
For example, sulphurous acid \[{H_2}S{O_3}\] .
Sulphuric acid group- these are also called oil of vitriol, produced by lead chamber and contact processes by dissolving \[S{O_3}\] in water. For example, sulphuric acid \[{H_2}S{O_4}\] (oleum) and thiosulphuric acid.
Thionic acid group- these are a series of unstable acids with general formula of \[{H_2}{S_n}{O_6}\] where \[n = 2\] to 6. For example, Dithionic acid \[{H_2}{S_2}{O_6}\] .
Peroxo acid group- these are also called Marshall’s acid or Caro’s acid. The central atom sulphur has +6 oxidation state with a peroxo group in it. These are derived from hydrogen peroxide by replacing hydrogen atoms. For example, peroxydisulfuric acid \[{H_2}{S_2}{O_8}\] .
From all these series, we saw that the \[S - S\] bond is present only in thionic acid series. For example, dithionic acid \[{H_2}{S_2}{O_6}\] .
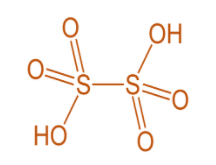
Hence, the correct option is (C).
Note: All oxyacids have the acidic hydrogen bonded to an oxygen atom. i.e. \[ - OH\] bond. The electronegativity of the central atom and the number of O atoms are responsible for the oxyacid acidity.
Complete step-by-step solution:
The sulphur oxyacids are the acids that contain oxygen, hydrogen and sulphur. These are divides into four groups based on their structural similarities:
Sulphurous acid group- these are prepared by dissolving sulphur dioxide in water. Their structure is pyramidal with three oxygen atoms on a triangle i.e. tetrahedral structure is distorted by lone pairs. These are strong reducing agents and have bleaching properties.
For example, sulphurous acid \[{H_2}S{O_3}\] .
Sulphuric acid group- these are also called oil of vitriol, produced by lead chamber and contact processes by dissolving \[S{O_3}\] in water. For example, sulphuric acid \[{H_2}S{O_4}\] (oleum) and thiosulphuric acid.
Thionic acid group- these are a series of unstable acids with general formula of \[{H_2}{S_n}{O_6}\] where \[n = 2\] to 6. For example, Dithionic acid \[{H_2}{S_2}{O_6}\] .
Peroxo acid group- these are also called Marshall’s acid or Caro’s acid. The central atom sulphur has +6 oxidation state with a peroxo group in it. These are derived from hydrogen peroxide by replacing hydrogen atoms. For example, peroxydisulfuric acid \[{H_2}{S_2}{O_8}\] .
From all these series, we saw that the \[S - S\] bond is present only in thionic acid series. For example, dithionic acid \[{H_2}{S_2}{O_6}\] .

Hence, the correct option is (C).
Note: All oxyacids have the acidic hydrogen bonded to an oxygen atom. i.e. \[ - OH\] bond. The electronegativity of the central atom and the number of O atoms are responsible for the oxyacid acidity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




