
Which one of the following will most readily be dehydrated in an acidic medium?
A.
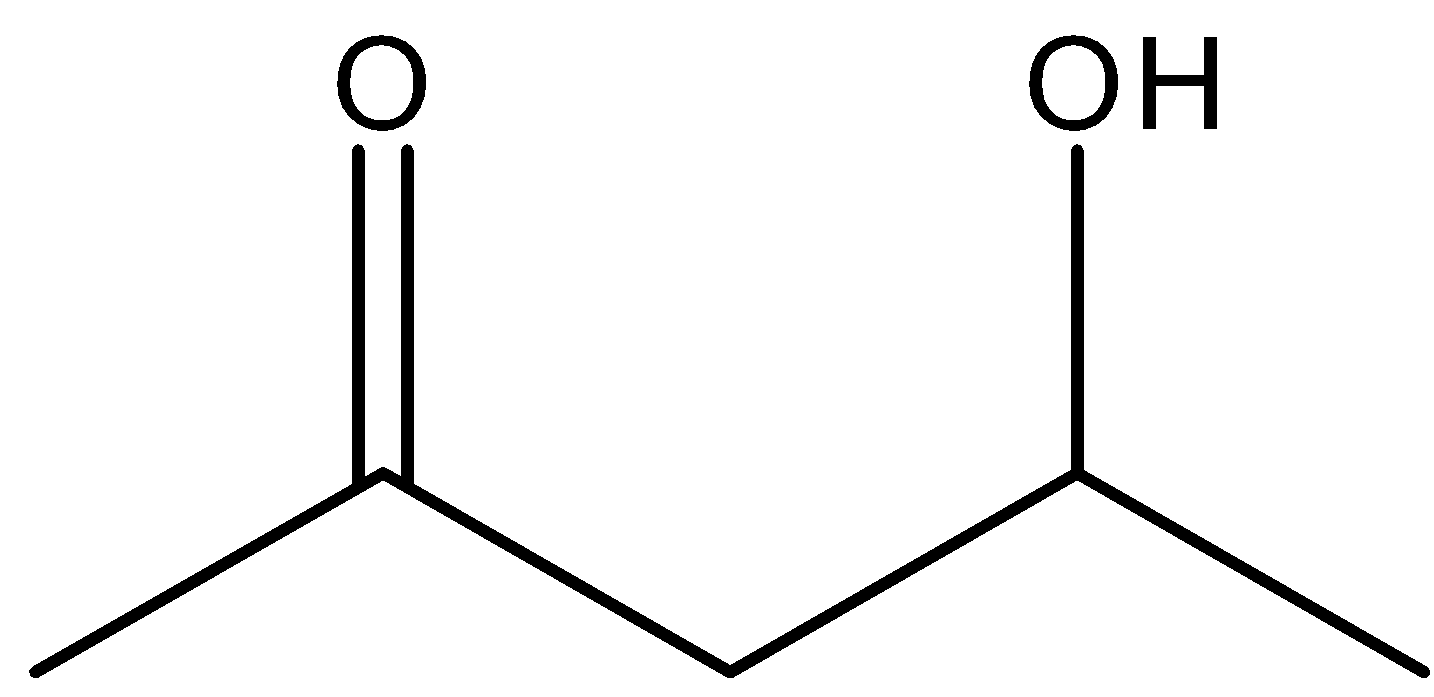
B.
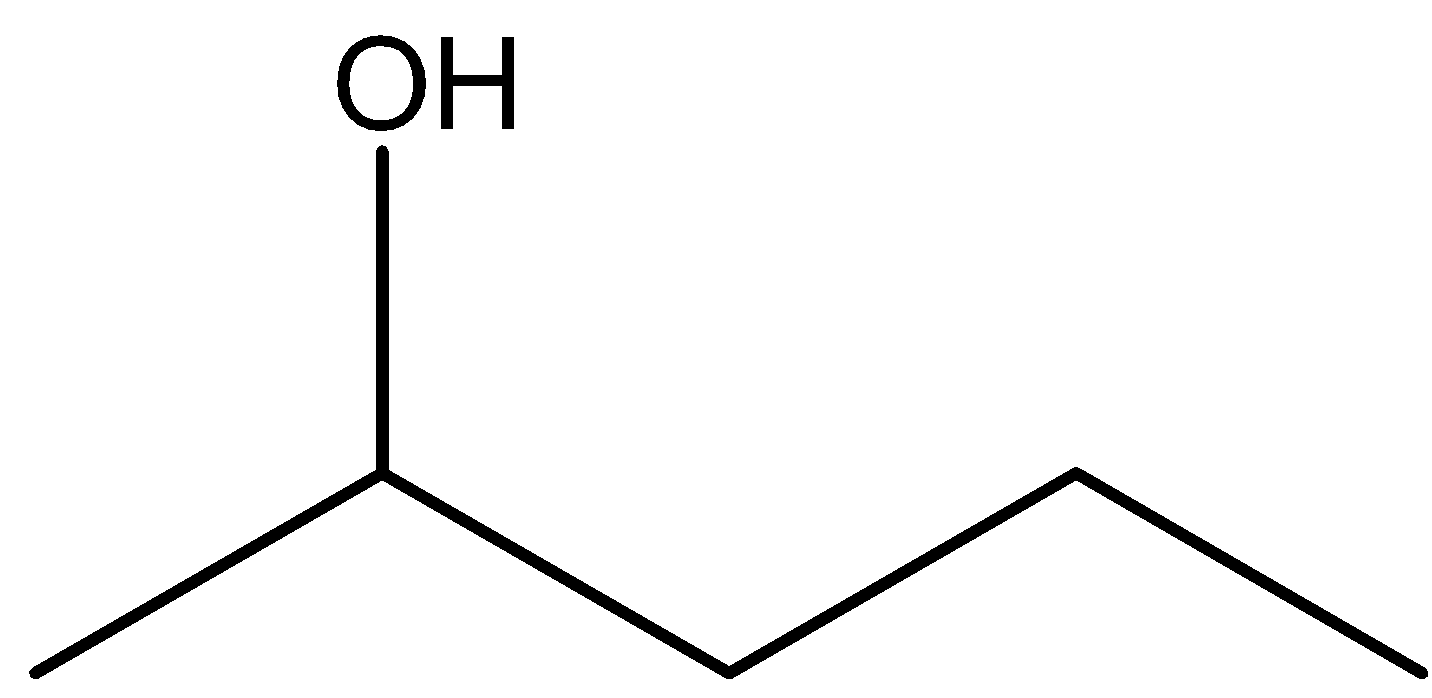
C.
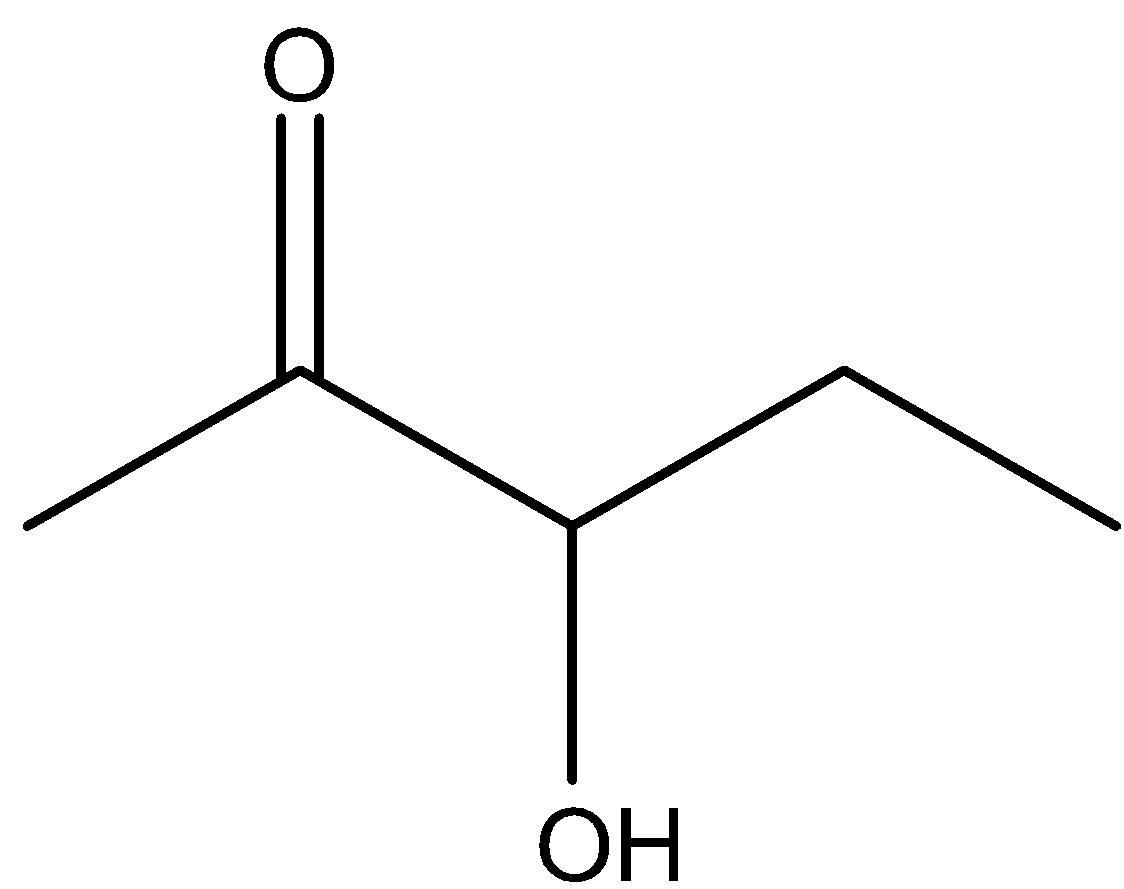
D.
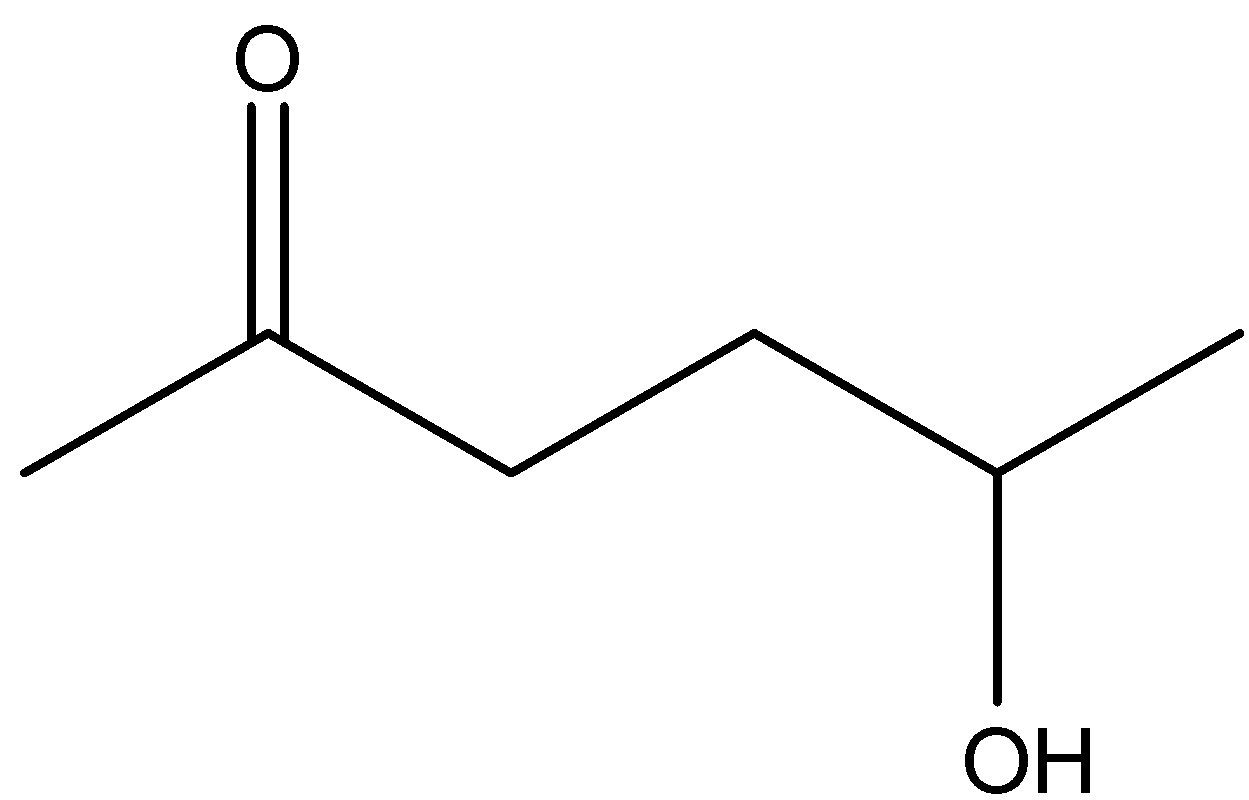




Answer
510.6k+ views
Hint: We have to remember that during the dehydration reaction, there occurs a conversion of reactant to the product by the loss of water. The dehydration reaction is the opposite reaction of the hydration reaction. Here, two compounds are reacted together and there is a formation of water as one product. For example, by the dehydration of alcohol there is a formation of alkene with elimination of water.
Complete answer:
The given reactant is \[4 - \]hydroxypentan\[ - 2 - \]one. And it is most readily dehydrated in acidic medium. Because, the dehydration power increases with increase the conjugation and stability of the compound. And in the case of \[4 - \]hydroxypentan\[ - 2 - \]one, the conjugation is high and the stability also very high. Therefore, in acidic medium it is most readily dehydrated and there is a formation of more stable conjugated alkene with water.
Let’s see the reaction,
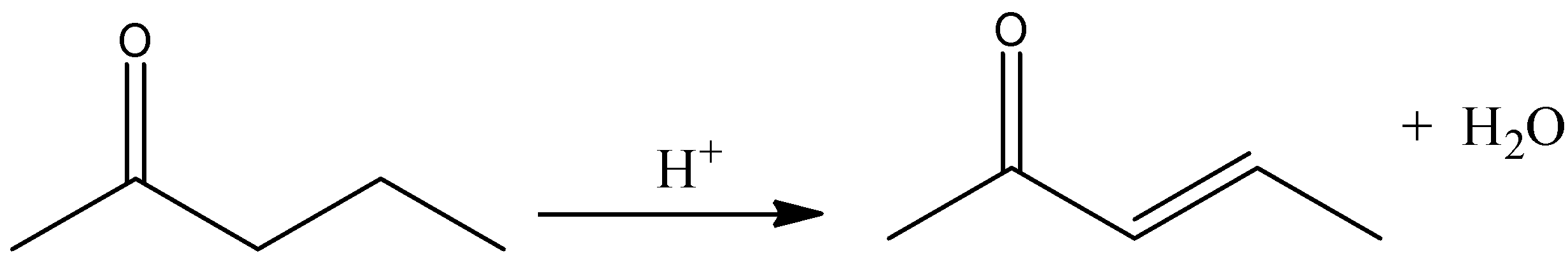
Hence, option (A) is correct.
Pentan \[ - 2 - \] ol is not most readily dehydrated in the presence of acidic medium. Hence, the option (B) is incorrect.
\[3 - \] Hydroxypentan \[ - 2 - \] one is not readily reacted with acidic medium and it will not dehydrate. Hence, option (C) is incorrect.
\[5 - \] Hydroxyhexan \[ - 2 - \] one is not a stable compound and it will not readily dehydrate with acidic medium. Hence, the option (D) is incorrect.
Hence, option (A) is correct.
Note:
We need to know that the dehydration reaction mainly depends on conjugation and stability. The power dehydration reaction is directly proportional to the conjugation and stability of the compound. If the compound is more stable, it has higher conjugation. Accordingly, dehydration also increases. The dehydration of alcohol gives more conjugated alkene with the water molecules.
Complete answer:
The given reactant is \[4 - \]hydroxypentan\[ - 2 - \]one. And it is most readily dehydrated in acidic medium. Because, the dehydration power increases with increase the conjugation and stability of the compound. And in the case of \[4 - \]hydroxypentan\[ - 2 - \]one, the conjugation is high and the stability also very high. Therefore, in acidic medium it is most readily dehydrated and there is a formation of more stable conjugated alkene with water.
Let’s see the reaction,

Hence, option (A) is correct.
Pentan \[ - 2 - \] ol is not most readily dehydrated in the presence of acidic medium. Hence, the option (B) is incorrect.
\[3 - \] Hydroxypentan \[ - 2 - \] one is not readily reacted with acidic medium and it will not dehydrate. Hence, option (C) is incorrect.
\[5 - \] Hydroxyhexan \[ - 2 - \] one is not a stable compound and it will not readily dehydrate with acidic medium. Hence, the option (D) is incorrect.
Hence, option (A) is correct.
Note:
We need to know that the dehydration reaction mainly depends on conjugation and stability. The power dehydration reaction is directly proportional to the conjugation and stability of the compound. If the compound is more stable, it has higher conjugation. Accordingly, dehydration also increases. The dehydration of alcohol gives more conjugated alkene with the water molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




