
Which one of the following statements is True:
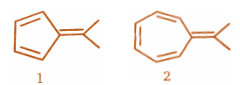
(A) \[PhLi\] adds to both compounds with equal ease
(B) \[PhLi\] does not add to either of the compounds
(C) \[PhLi\] reacts readily with 1 but does not add to 2
(D) \[PhLi\] reacts readily with 2 but does not add to 1

Answer
559.2k+ views
Hint:To solve this question, we must first some basic knowledge about Phenyllithium $(PhLi)$ and also we must understand the basic concepts of Aromaticity. Then we need to assess the properties of \[PhLi\] and then use the rules for Aromaticity to determine that what will be the action of \[PhLi\] on $1\,\,and\,\,2$ and then only we can conclude the correct answer.
Complete step-by-step answer: \[PhLi\] : Phenyllithium or lithobenzene is an organometallic agent with the empirical formula ${C_6}{H_5}Li$ . It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses.Aromaticity: It is defined as a property of the conjugated cycloalkenes which enhances the stability of a molecule due to the delocalization of electrons present in the π-π orbitals.
The aromatics compounds are said to exhibit some special characteristics or called as rules which are given below-
1.Aromatic compounds are always cyclic structures.
2.Each element of the ring within the structure must and should have a p-orbital ring which is in a perpendicular form to the ring, and this makes it a planar molecule
3.All the compounds obey the Huckel’s Rule, i.e. all the aromatic compounds should have the \[\left( {4n + 2} \right)\pi \] number of electrons.
4.The organic compound has to be flat.
Step 1: For the first compound:
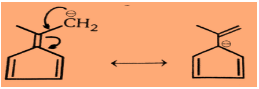
So very clearly we can see in the above structures that it is an aromatic compound.
Step2:For the second compound:
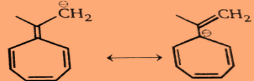
So very clearly we can see in the above structures that it is an antiaromatic compound.And Hence, the first compound will be more reactive towards \[PhLi\] and the second compound will be unreactive towards \[PhLi\] .Conclusion: \[PhLi\] reacts readily with 1 but does not add to 2
So, clearly we can conclude that the correct answer is Option (C).
Note:Crystalline phenyllithium is colorless; however, solutions of phenyllithium are various shades of brown or red depending on the solvent used and the impurities present in the solute.
Complete step-by-step answer: \[PhLi\] : Phenyllithium or lithobenzene is an organometallic agent with the empirical formula ${C_6}{H_5}Li$ . It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses.Aromaticity: It is defined as a property of the conjugated cycloalkenes which enhances the stability of a molecule due to the delocalization of electrons present in the π-π orbitals.
The aromatics compounds are said to exhibit some special characteristics or called as rules which are given below-
1.Aromatic compounds are always cyclic structures.
2.Each element of the ring within the structure must and should have a p-orbital ring which is in a perpendicular form to the ring, and this makes it a planar molecule
3.All the compounds obey the Huckel’s Rule, i.e. all the aromatic compounds should have the \[\left( {4n + 2} \right)\pi \] number of electrons.
4.The organic compound has to be flat.
Step 1: For the first compound:

So very clearly we can see in the above structures that it is an aromatic compound.
Step2:For the second compound:

So very clearly we can see in the above structures that it is an antiaromatic compound.And Hence, the first compound will be more reactive towards \[PhLi\] and the second compound will be unreactive towards \[PhLi\] .Conclusion: \[PhLi\] reacts readily with 1 but does not add to 2
So, clearly we can conclude that the correct answer is Option (C).
Note:Crystalline phenyllithium is colorless; however, solutions of phenyllithium are various shades of brown or red depending on the solvent used and the impurities present in the solute.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




