
Which one of the following reagent(s) is/are used for the conversion of ketone into hydrocarbons?
A) $ LiAl{H_4} $
B) $ {N_2}{H_4}/{H_2}{O_2} $
C) $ Mg/Hg,{H_2}O $
D) $ Na/Hg,HCl $
Answer
478.5k+ views
Hint: The process of conversion of ketones to hydrocarbons is a reduction process. Firstly, the reduction of ketones will give alcohol, and further removal of the hydroxyl group will give us Hydrocarbons. Commonly, Clemmensen Reduction is used for this reduction.
Complete answer:
Let us see the reactions of the given reagents on ketones one by one:
1. $ LiAl{H_4} $ : Lithium Aluminium Hydride is one of the most useful reducing agents in organic chemistry. It serves as a source of Hydride i.e. $ {H^ - } $ . The negative charge is due to the Hydrogen being more electronegative than Aluminium. The reaction of $ LiAl{H_4} $ on Ketones gives Secondary Alcohols. Hence option A is incorrect.
2. $ {N_2}{H_4}/{H_2}{O_2} $ : $ {N_2}{H_4}/{H_2}{O_2} $ is a strong reducing agents and used for the reduction of Alkenes and Alkynes. The hydrogen is added in a syn fashion to reduce the multiple bonds. $ {N_2}{H_4}/KOH $ is used in the Wolff-Kirshner reduction, which reduces carbonyls to alkanes. Option B is incorrect.
3. $ Mg/Hg,{H_2}O $ : The reaction of ketones with Mg-Hg is known as Pinacol Coupling reaction. It is a free radical process. The C-C bonds are formed between carbonyl groups of ketones. Leads to the formation of diols. Option C is incorrect.
4. $ Na/Hg,HCl $ : Sodium Amalgam (Na-Hg) along with HCl is a strong reducing reagent used for reduction of ketones to alkanes (hydrocarbons). If Zn-Hg is used it is known as Clemmensen Reduction. Option D is correct. The Reaction can be given as:
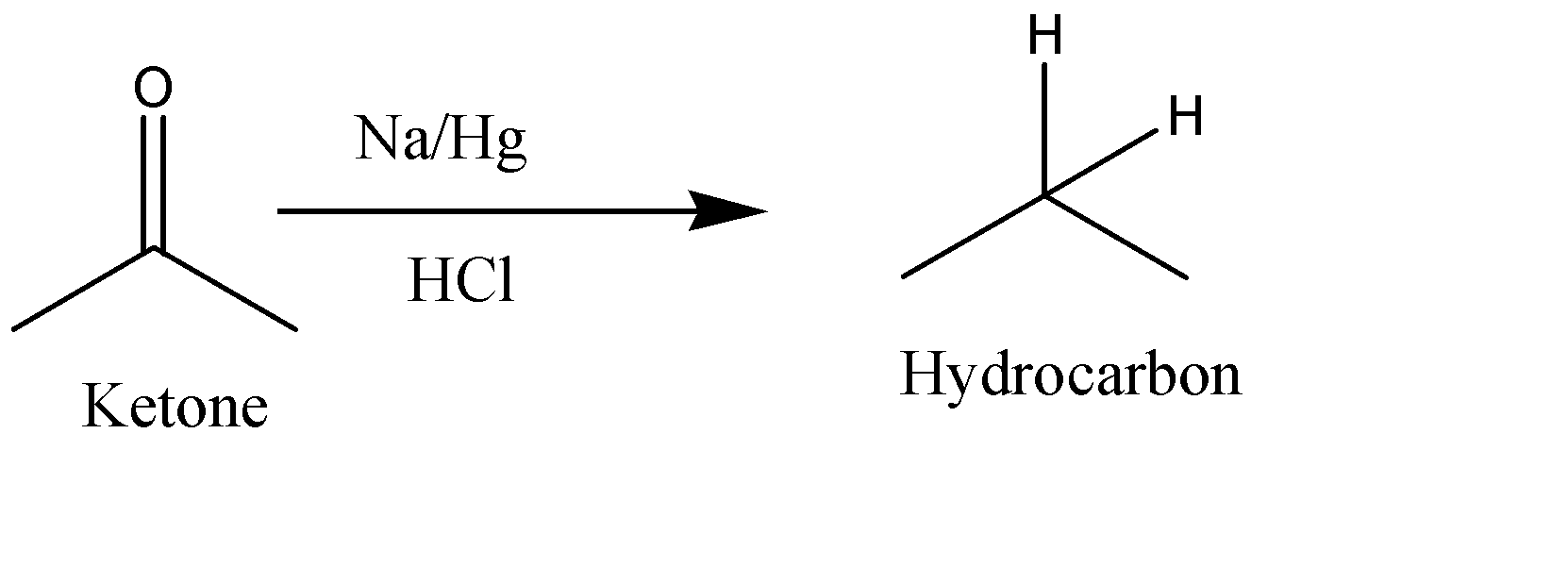
The correct answer is Option D.
Note:
Other reducing agents used for converting Carbonyls to Hydrocarbons are: Wolff-Kishner Reduction ( $ {N_2}{H_4}/KOH $ ), Clemmensen Reduction (Zn-Hg/ conc. HCl). Sodium Amalgams are also used in place of Zinc Amalgams.
Complete answer:
Let us see the reactions of the given reagents on ketones one by one:
1. $ LiAl{H_4} $ : Lithium Aluminium Hydride is one of the most useful reducing agents in organic chemistry. It serves as a source of Hydride i.e. $ {H^ - } $ . The negative charge is due to the Hydrogen being more electronegative than Aluminium. The reaction of $ LiAl{H_4} $ on Ketones gives Secondary Alcohols. Hence option A is incorrect.
2. $ {N_2}{H_4}/{H_2}{O_2} $ : $ {N_2}{H_4}/{H_2}{O_2} $ is a strong reducing agents and used for the reduction of Alkenes and Alkynes. The hydrogen is added in a syn fashion to reduce the multiple bonds. $ {N_2}{H_4}/KOH $ is used in the Wolff-Kirshner reduction, which reduces carbonyls to alkanes. Option B is incorrect.
3. $ Mg/Hg,{H_2}O $ : The reaction of ketones with Mg-Hg is known as Pinacol Coupling reaction. It is a free radical process. The C-C bonds are formed between carbonyl groups of ketones. Leads to the formation of diols. Option C is incorrect.
4. $ Na/Hg,HCl $ : Sodium Amalgam (Na-Hg) along with HCl is a strong reducing reagent used for reduction of ketones to alkanes (hydrocarbons). If Zn-Hg is used it is known as Clemmensen Reduction. Option D is correct. The Reaction can be given as:

The correct answer is Option D.
Note:
Other reducing agents used for converting Carbonyls to Hydrocarbons are: Wolff-Kishner Reduction ( $ {N_2}{H_4}/KOH $ ), Clemmensen Reduction (Zn-Hg/ conc. HCl). Sodium Amalgams are also used in place of Zinc Amalgams.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




