
Which one of the following organohalogen compounds when heated with alcoholic potassium hydroxide does not undergo dehydrohalogenation reaction?
(a)- Secondary butyl chloride
(b)- Isopropyl chloride
(c)- Neopentyl chloride
(d)- Isobutyl chloride
(e)- Tertiary butyl chloride
Answer
513.3k+ views
Hint: Dehydrohalogenation reaction means from the given compound, the halogen from one carbon atom and hydrogen from the adjacent carbon atom will be removed and this will form alkene. For this reaction to occur, the adjacent carbon must have a hydrogen atom.
Complete answer:
There are many types of organic reactions like halogenations reaction, addition reaction, hydrogenation reaction, dehydration reaction, dehydrohalogenation reaction, etc. Dehydrohalogenation reaction means from the given compound, the halogen from one carbon atom and hydrogen from the adjacent carbon atom will be removed and this will form alkene. For this reaction to occur, the adjacent carbon must have a hydrogen atom.
So, let us check all the structures of the compounds given in the options.
(a)- Secondary butyl chloride.
This is a compound of four carbon atoms and chlorine is present at the second carbon atom. The structure is:
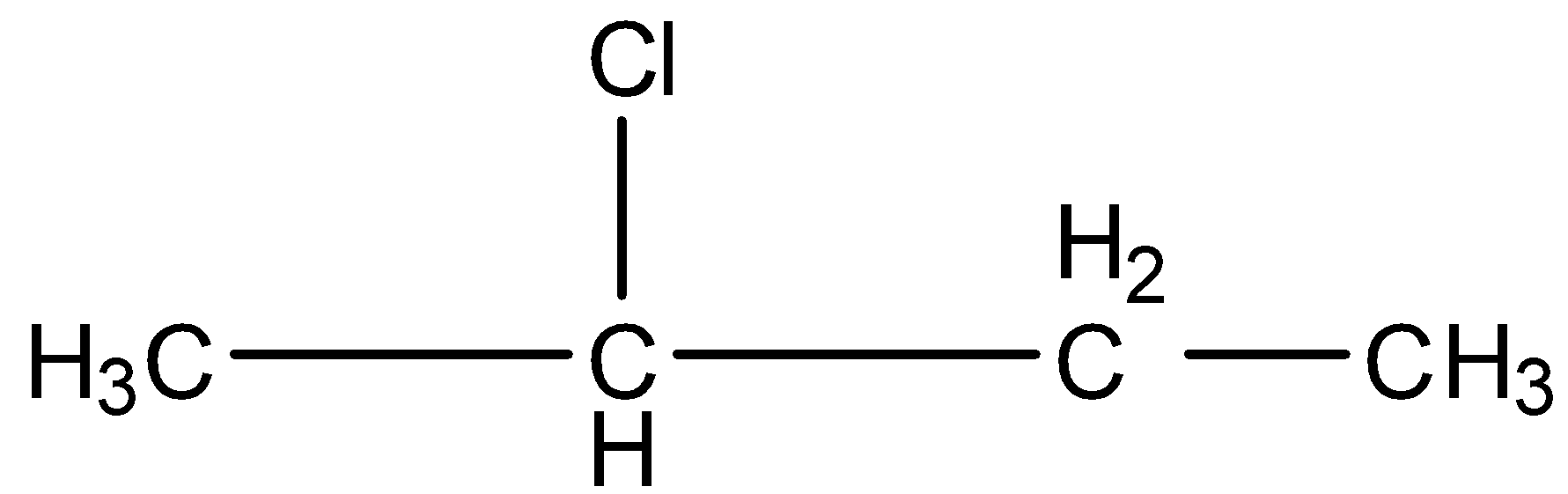
There are two carbon atoms attached to the carbon atom having the chlorine atom. Both the carbon atoms have hydrogen atoms so, the dehydrohalogenation reaction will occur.
(b)- Isopropyl chloride
This is a compound of three carbon atoms and chlorine is present at the second carbon atom. The structure is:
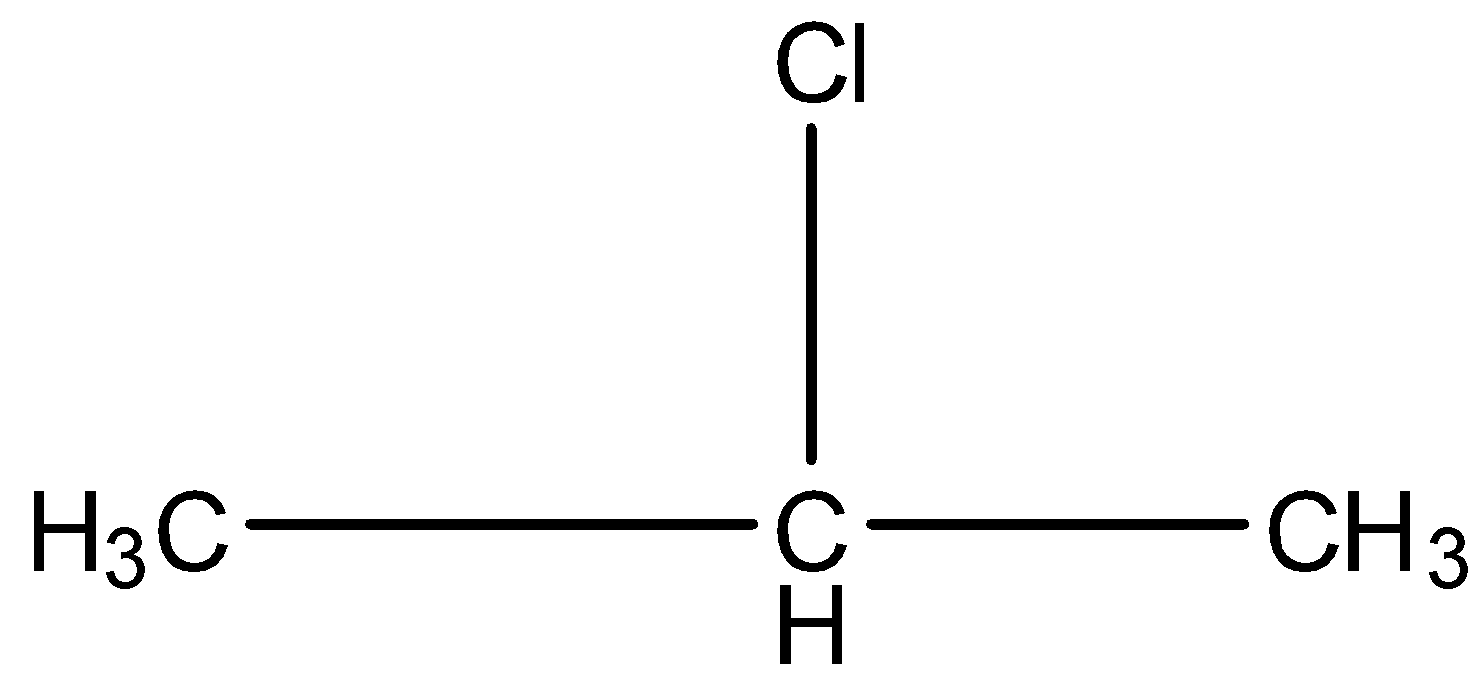
There are two carbon atoms attached to the carbon atom having the chlorine atom. Both the carbon atoms have hydrogen atoms so, the dehydrohalogenation reaction will occur.
(c)- Neopentyl chloride
This is a compound of five carbon atoms and chlorine is present at the first carbon atom. The structure is:
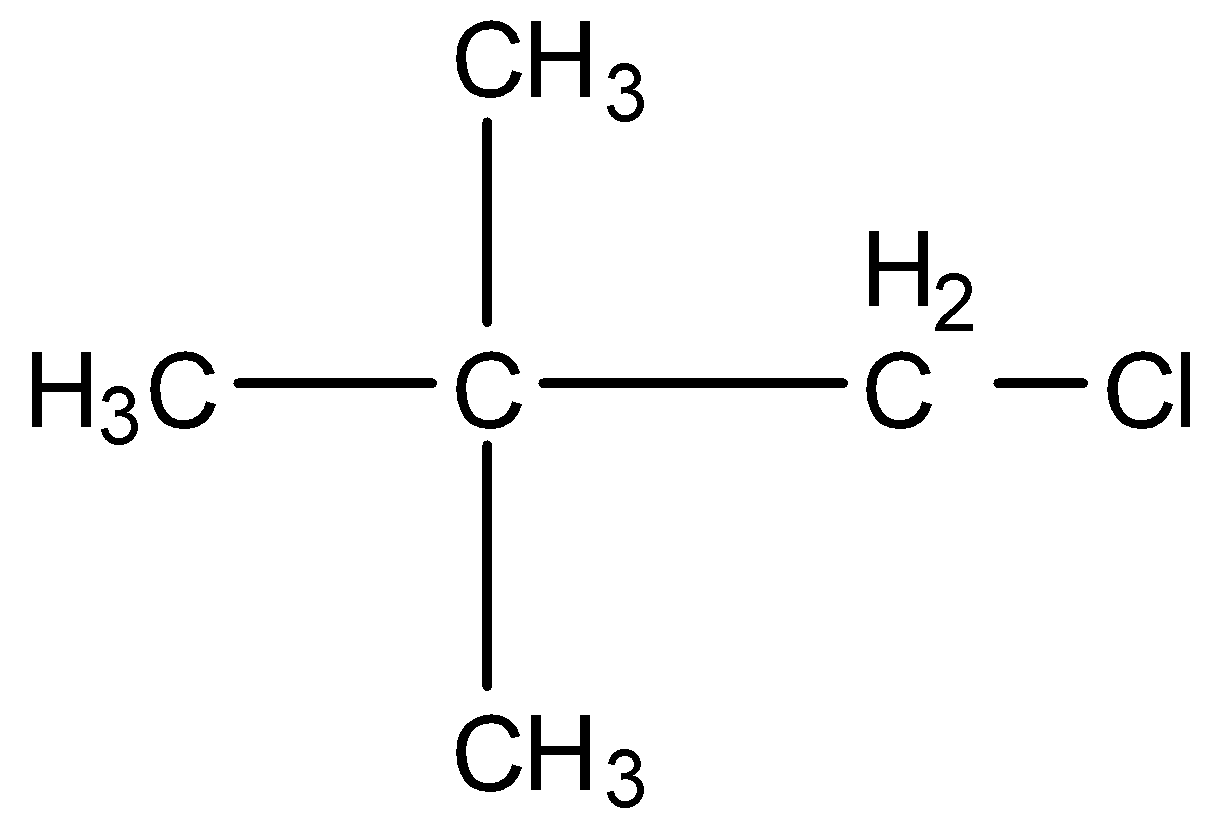
The carbon atom attached to the carbon atom having the chlorine atom doesn't have any hydrogen atom. So, dehydrohalogenation will not occur.
(d)- Isobutyl chloride
This is a compound of four carbon atoms and chlorine is present at the first carbon atom. The structure is:
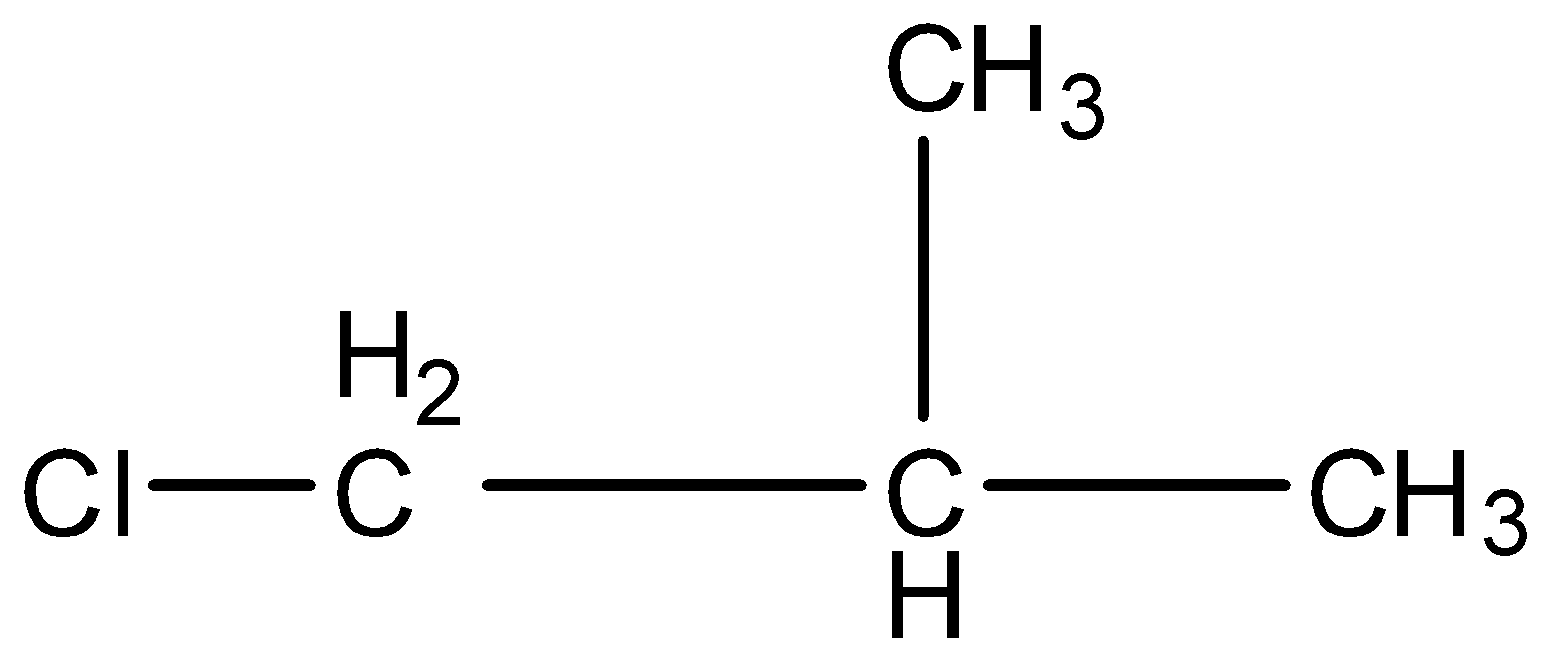
There is one carbon atom attached to the carbon atom having the chlorine atom. The carbon atom has a hydrogen atom so the dehydrohalogenation reaction will occur.
(e)- Tertiary butyl chloride
This is a compound of four carbon atoms and chlorine is present at the first carbon atom. The structure is:
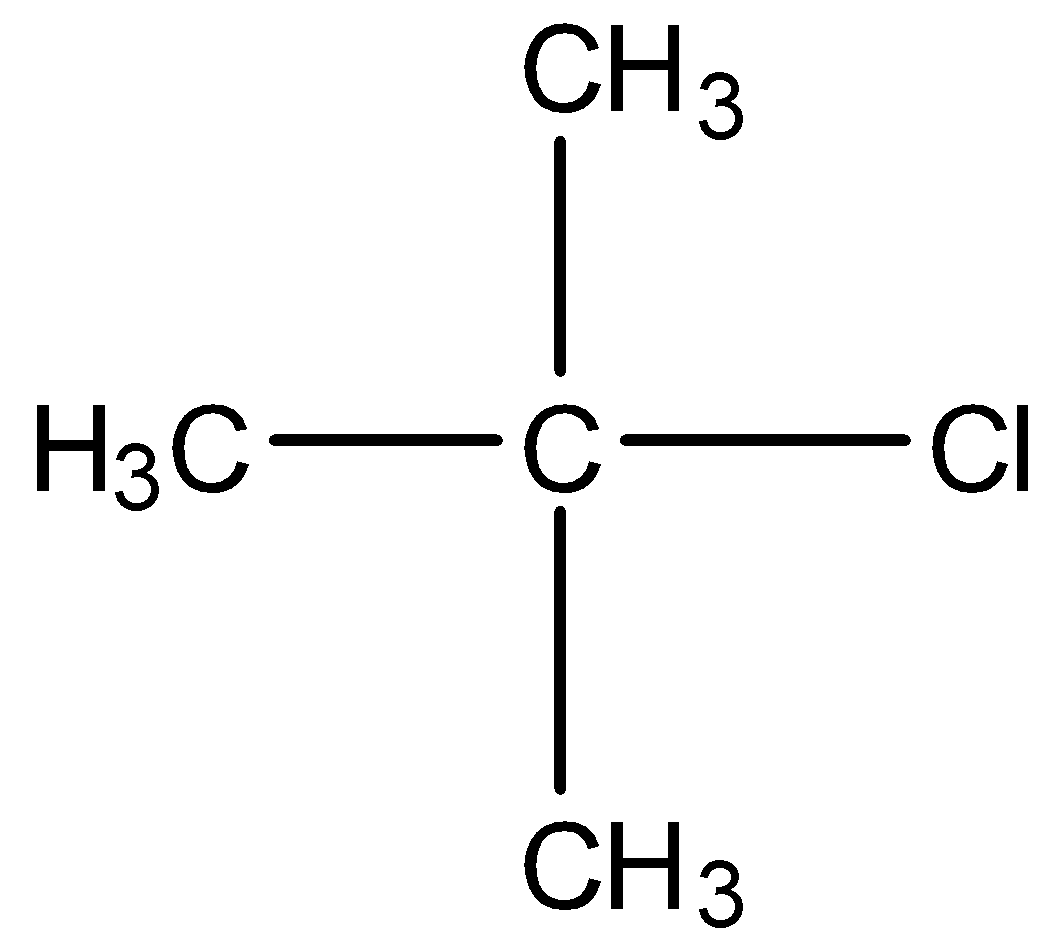
There are three carbon atoms attached to the carbon atom having the chlorine atom. All the carbon atoms have hydrogen atoms so, the dehydrohalogenation reaction will occur.
Therefore, the correct answer is an option (c).
Note:
The carbon atom having the chlorine atom or halogen atom is called an alpha carbon atom and the adjacent carbon atoms are known as a beta-carbon atom. The necessary condition for the dehydrohalogenation reaction to occur is the presence of alcoholic potassium hydroxide.
Complete answer:
There are many types of organic reactions like halogenations reaction, addition reaction, hydrogenation reaction, dehydration reaction, dehydrohalogenation reaction, etc. Dehydrohalogenation reaction means from the given compound, the halogen from one carbon atom and hydrogen from the adjacent carbon atom will be removed and this will form alkene. For this reaction to occur, the adjacent carbon must have a hydrogen atom.
So, let us check all the structures of the compounds given in the options.
(a)- Secondary butyl chloride.
This is a compound of four carbon atoms and chlorine is present at the second carbon atom. The structure is:

There are two carbon atoms attached to the carbon atom having the chlorine atom. Both the carbon atoms have hydrogen atoms so, the dehydrohalogenation reaction will occur.
(b)- Isopropyl chloride
This is a compound of three carbon atoms and chlorine is present at the second carbon atom. The structure is:

There are two carbon atoms attached to the carbon atom having the chlorine atom. Both the carbon atoms have hydrogen atoms so, the dehydrohalogenation reaction will occur.
(c)- Neopentyl chloride
This is a compound of five carbon atoms and chlorine is present at the first carbon atom. The structure is:

The carbon atom attached to the carbon atom having the chlorine atom doesn't have any hydrogen atom. So, dehydrohalogenation will not occur.
(d)- Isobutyl chloride
This is a compound of four carbon atoms and chlorine is present at the first carbon atom. The structure is:

There is one carbon atom attached to the carbon atom having the chlorine atom. The carbon atom has a hydrogen atom so the dehydrohalogenation reaction will occur.
(e)- Tertiary butyl chloride
This is a compound of four carbon atoms and chlorine is present at the first carbon atom. The structure is:

There are three carbon atoms attached to the carbon atom having the chlorine atom. All the carbon atoms have hydrogen atoms so, the dehydrohalogenation reaction will occur.
Therefore, the correct answer is an option (c).
Note:
The carbon atom having the chlorine atom or halogen atom is called an alpha carbon atom and the adjacent carbon atoms are known as a beta-carbon atom. The necessary condition for the dehydrohalogenation reaction to occur is the presence of alcoholic potassium hydroxide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




