
Which one of the following nitro-compounds does not react with nitrous acid.
A.
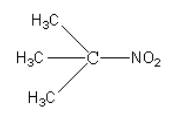
B.
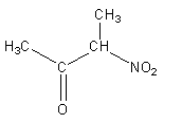
C.

D.
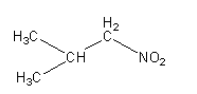




Answer
572.1k+ views
Hint:The nitro compound having alpha hydrogen reacts with nitrous acid. The reaction is used for distinguished primary, secondary and tertiary nitro compounds.
Complete answer:
The reaction of nitrous acid with different nitro alkane gives different results, so this is used to differentiate the primary, secondary and tertiary nitro compounds.
The product of all nitroalkanes with nitrous acid is shown as follows:
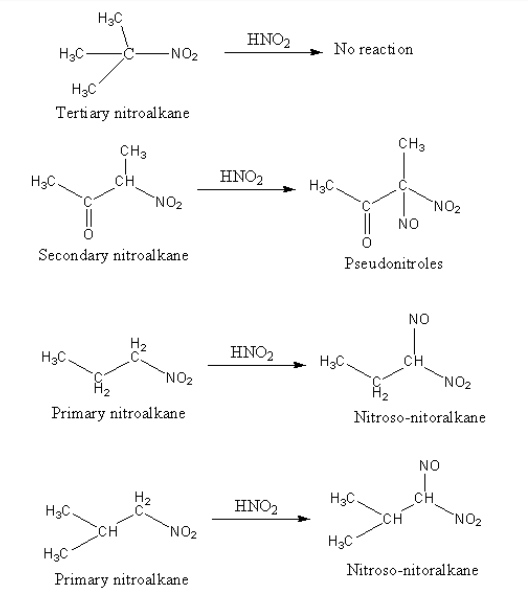
The carbon directly attached to the nitro group is known as alpha carbon and the hydrogen attached with alpha carbon is known as alpha hydrogen. Based on the number of carbon attached with alpha carbon, the nitroalkane is differentiated as primary, secondary and tertiary. If the alpha carbon is attached with only one carbon the nitroalkane is known as primary nitroalkane.
Primary nitroalkane has alpha hydrogen, so they react with nitrous acid and form nitroso-nitroalkane.
Secondary nitroalkane also has alpha hydrogen, so they react with nitrous acid and form pseudonitroles.
Tertiary nitroalkanes do not have alpha hydrogen, so they do not react with nitrous acid.
Nitroalkane in option (A) is tertiary nitroalkane, so it does not react with nitrous acid, so option (A) is correct.
Nitroalkane in option (B) is secondary and nitroalkane in option (C) and (D) are primary nitroalkane, so they react with nitrous acid.
Therefore, option (A) $({CH_3})_3CNO_2 $ is correct.
Note:
The nitroso-nitroalkane gives blue colour in sodium hydroxide solution whereas the pseudonitroles give red colour in sodium hydroxide solution and tertiary nitroalkane does not react with nitrous acid.
Complete answer:
The reaction of nitrous acid with different nitro alkane gives different results, so this is used to differentiate the primary, secondary and tertiary nitro compounds.
The product of all nitroalkanes with nitrous acid is shown as follows:

The carbon directly attached to the nitro group is known as alpha carbon and the hydrogen attached with alpha carbon is known as alpha hydrogen. Based on the number of carbon attached with alpha carbon, the nitroalkane is differentiated as primary, secondary and tertiary. If the alpha carbon is attached with only one carbon the nitroalkane is known as primary nitroalkane.
Primary nitroalkane has alpha hydrogen, so they react with nitrous acid and form nitroso-nitroalkane.
Secondary nitroalkane also has alpha hydrogen, so they react with nitrous acid and form pseudonitroles.
Tertiary nitroalkanes do not have alpha hydrogen, so they do not react with nitrous acid.
Nitroalkane in option (A) is tertiary nitroalkane, so it does not react with nitrous acid, so option (A) is correct.
Nitroalkane in option (B) is secondary and nitroalkane in option (C) and (D) are primary nitroalkane, so they react with nitrous acid.
Therefore, option (A) $({CH_3})_3CNO_2 $ is correct.
Note:
The nitroso-nitroalkane gives blue colour in sodium hydroxide solution whereas the pseudonitroles give red colour in sodium hydroxide solution and tertiary nitroalkane does not react with nitrous acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




