
Which one of the following is not a Lewis acid?
A. $B{F_3}$
B. $AlC{l_3}$
C. $BeC{l_2}$
D. $SnC{l_2}$
Answer
574.2k+ views
Hint: We can use the definition of Lewis acid to deduce which one of the given compounds cannot be classified as a Lewis acid. The electron deficient compounds are considered as Lewis acids.
Complete answer
We know that various efforts were made to explain the properties of matter with different theories for bonding between the atoms. One such theory was given by Lewis. He proposed that atoms combine to complete their octet and a bond is formed. We can take the example of formation of ${N_2}$. Here, we have two atoms, both of nitrogen. We can write the Lewis symbols for these by representing the valence electrons as dots:

As we can see that both the atoms have $5$ valence electrons and need $3$ more to complete their octet. In order to do that they share $3$ pairs of electrons that can be shown as follows:
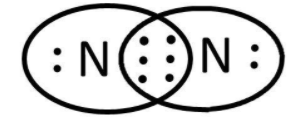
We know that this sharing of valence electrons result in covalent bond formation. So, the bonding can be shown as:
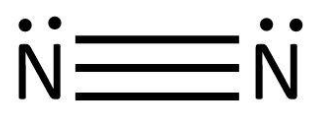
Lewis took it further and defined acids and bases as molecules which accept and donate electrons respectively while forming a bond. Based on this, we will examine the Lewis structures of the given molecules and try to deduce whether they are Lewis acids or not.
$B{F_3}$: here, we have $3$ and $7$ valence electrons from $B$ and $F$ respectively. Let’s write its Lewis structure:
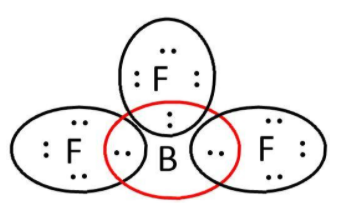
As we can see that octet of all $3\;F$ are complete but not that of boron. So, it is electron deficient and can act as a Lewis acid.
$AlC{l_3}$: here, we have $3$ and $7$ valence electrons from $Al$ and $Cl$ respectively. Let’s write its Lewis structure:
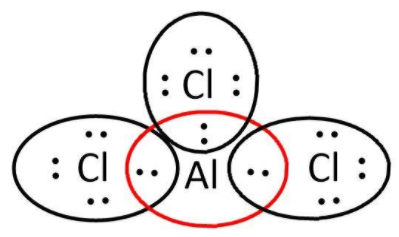
As we can see that octets of all $3\;Cl$ are complete but not that of aluminum. So, it is electron deficient and can act as a Lewis acid.
$BeC{l_2}$: here, we have $2$ and $7$ valence electrons from $Be$ and $Cl$ respectively. Let’s write its Lewis structure:
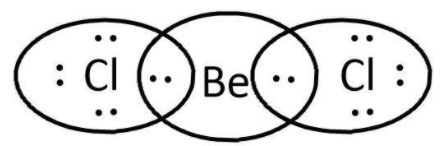
As we can see that octets of both $Cl$ are complete and $Be$ cannot accommodate more electrons as well for its valence orbital is filled. So, it is not electron deficient and cannot act as a Lewis acid.
$SnC{l_2}$: here, we have $4$ and $7$ valence electrons from $Sn$ and $Cl$ respectively. Let’s write its Lewis structure:
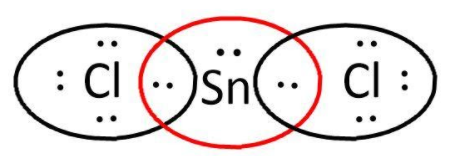
As we can see that octets of both $Cl$ are complete but not that of $Sn$ and it can also use its d-orbitals. So, it is electron deficient and can act as a Lewis acid.
Hence, the correct option is C.
Note:
There are some molecules that do not follow the octet rule such as $BeC{l_2}$ so we can’t rely solely on octet rule for electron deficiency.
Complete answer
We know that various efforts were made to explain the properties of matter with different theories for bonding between the atoms. One such theory was given by Lewis. He proposed that atoms combine to complete their octet and a bond is formed. We can take the example of formation of ${N_2}$. Here, we have two atoms, both of nitrogen. We can write the Lewis symbols for these by representing the valence electrons as dots:

As we can see that both the atoms have $5$ valence electrons and need $3$ more to complete their octet. In order to do that they share $3$ pairs of electrons that can be shown as follows:

We know that this sharing of valence electrons result in covalent bond formation. So, the bonding can be shown as:
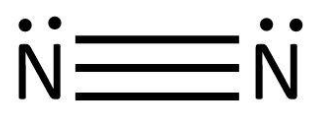
Lewis took it further and defined acids and bases as molecules which accept and donate electrons respectively while forming a bond. Based on this, we will examine the Lewis structures of the given molecules and try to deduce whether they are Lewis acids or not.
$B{F_3}$: here, we have $3$ and $7$ valence electrons from $B$ and $F$ respectively. Let’s write its Lewis structure:

As we can see that octet of all $3\;F$ are complete but not that of boron. So, it is electron deficient and can act as a Lewis acid.
$AlC{l_3}$: here, we have $3$ and $7$ valence electrons from $Al$ and $Cl$ respectively. Let’s write its Lewis structure:

As we can see that octets of all $3\;Cl$ are complete but not that of aluminum. So, it is electron deficient and can act as a Lewis acid.
$BeC{l_2}$: here, we have $2$ and $7$ valence electrons from $Be$ and $Cl$ respectively. Let’s write its Lewis structure:

As we can see that octets of both $Cl$ are complete and $Be$ cannot accommodate more electrons as well for its valence orbital is filled. So, it is not electron deficient and cannot act as a Lewis acid.
$SnC{l_2}$: here, we have $4$ and $7$ valence electrons from $Sn$ and $Cl$ respectively. Let’s write its Lewis structure:

As we can see that octets of both $Cl$ are complete but not that of $Sn$ and it can also use its d-orbitals. So, it is electron deficient and can act as a Lewis acid.
Hence, the correct option is C.
Note:
There are some molecules that do not follow the octet rule such as $BeC{l_2}$ so we can’t rely solely on octet rule for electron deficiency.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




