
Which one of the following is more acidic?


Answer
591.9k+ views
Hint: The strength of an acid depends on the ability of acid to donate the proton and stability of the conjugate base. Electron donating group decreases the acidity of acid and electron-withdrawing group increases the acidity of acid.
Step by step answer: The strength of an acid depends on the ability of acid to donate the proton. Acids having a greater ability to donate the protons are known as a strong acid.
Out of the given acids compound given in option A is alcohol. Alcohols are weaker acid than the phenol as the conjugate base of phenol is stabilized by resonance structure.
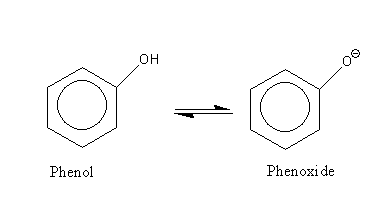
Resonance structures of phenol are as follows:
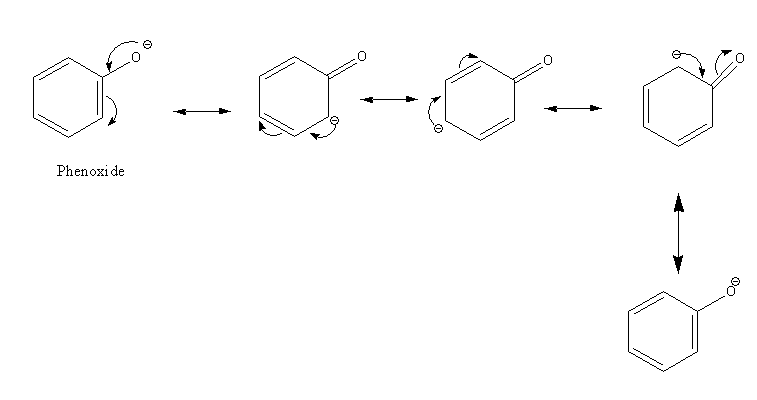
The acidic strength of substituted phenol is dependent on the nature of the substituent.
The electron-withdrawing group decreases the electron density of the phenol ring and stabilizes the negative charge and thus increases the acid strength.
In the case of the compound given in option B (ortho-nitro phenol) the substituent is a nitro group (${\text{ - N}}{{\text{O}}_{\text{2}}}$ ). It is an electron-withdrawing group; it increases the acidity so ortho-nitro phenol is a stronger acid than phenol.
Electron donating substituent increases the electron density of the phenol ring and destabilizes the negative charge and thus decreases the acidity.
In the case of the compound given in option D (ortho-cresol) the substituent is a methyl group (${\text{ - C}}{{\text{H}}_{\text{3}}}$ ). It is an electron-donating group, it decreases the acidity so ortho-cresol is a weaker acid than phenol.
So the decreasing order of acid strength of a given compound is as follows:
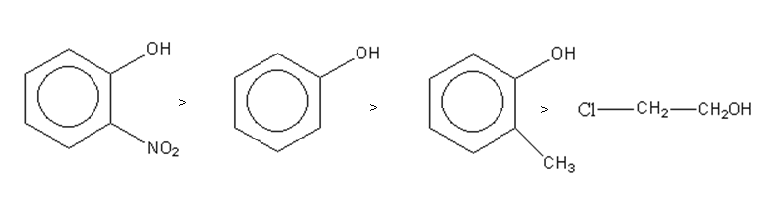
So, option (B) is the correct answer.
Note: Ring activating groups that are electron-donating groups decreases the strength of acid while a ring deactivating group increases the strength of the acid. Alkyls are electron-donating groups. Halides and nitro groups are electron-withdrawing groups.
Step by step answer: The strength of an acid depends on the ability of acid to donate the proton. Acids having a greater ability to donate the protons are known as a strong acid.
Out of the given acids compound given in option A is alcohol. Alcohols are weaker acid than the phenol as the conjugate base of phenol is stabilized by resonance structure.
Resonance structures of phenol are as follows:


The acidic strength of substituted phenol is dependent on the nature of the substituent.
The electron-withdrawing group decreases the electron density of the phenol ring and stabilizes the negative charge and thus increases the acid strength.
In the case of the compound given in option B (ortho-nitro phenol) the substituent is a nitro group (${\text{ - N}}{{\text{O}}_{\text{2}}}$ ). It is an electron-withdrawing group; it increases the acidity so ortho-nitro phenol is a stronger acid than phenol.
Electron donating substituent increases the electron density of the phenol ring and destabilizes the negative charge and thus decreases the acidity.
In the case of the compound given in option D (ortho-cresol) the substituent is a methyl group (${\text{ - C}}{{\text{H}}_{\text{3}}}$ ). It is an electron-donating group, it decreases the acidity so ortho-cresol is a weaker acid than phenol.
So the decreasing order of acid strength of a given compound is as follows:

So, option (B) is the correct answer.
Note: Ring activating groups that are electron-donating groups decreases the strength of acid while a ring deactivating group increases the strength of the acid. Alkyls are electron-donating groups. Halides and nitro groups are electron-withdrawing groups.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Coming together federation is practiced in A India class 12 social science CBSE

Write the formula to find the shortest distance between class 12 maths CBSE




