
Which one of the following has zero dipole moment?
A. $B{F_3}$
B.BeClBr
C. $C{H_2}C{l_2}$
D.COS
Answer
582.3k+ views
Hint: The $B{F_3}$ molecule has a symmetrical trigonal planar geometry. In such a structure, the resultant moment of any two \[B - F\] dipoles are equal in magnitude but opposite to the moment of the third one.
Complete step by step answer:
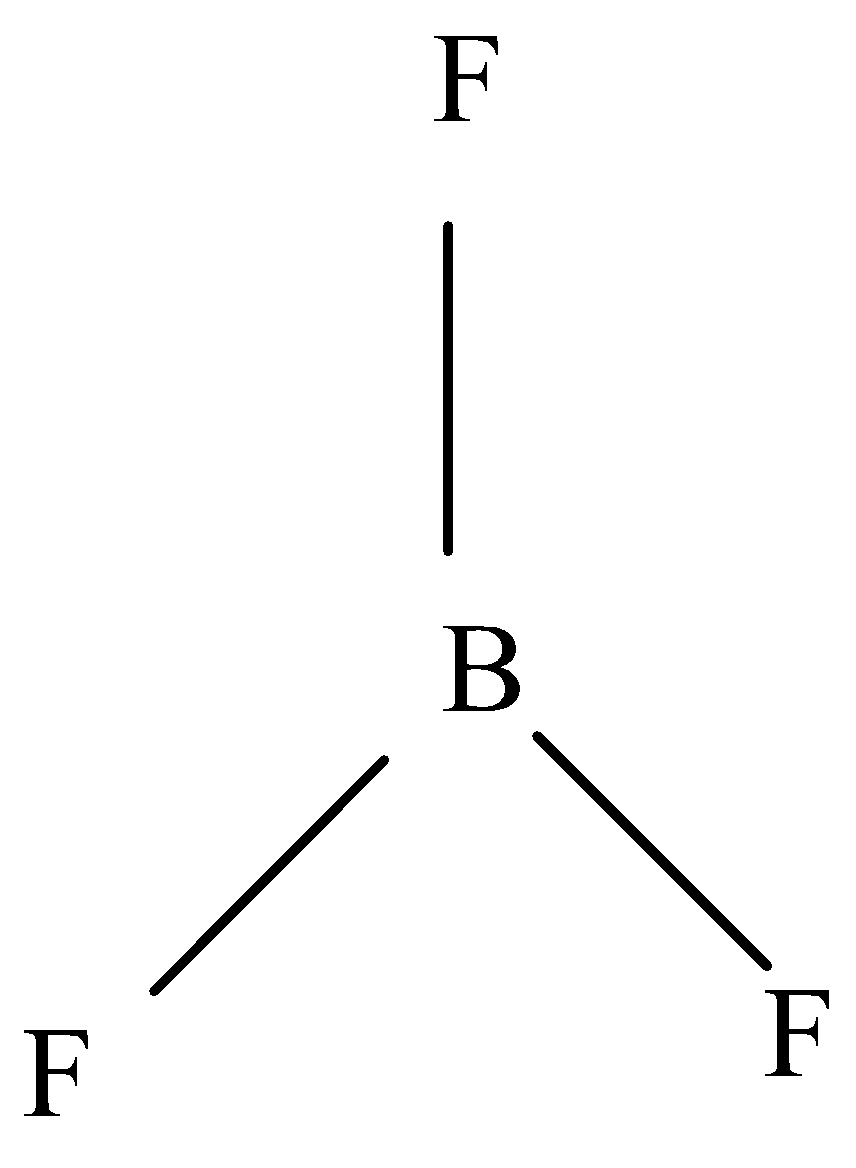
-In $B{F_3}$ (Boron trifluoride), its shape is trigonal planar, three fluorine are attached to the boron. It has no lone pairs of electrons on B. The three F are attached to B that the resultant dipole due to two \[B - F\] bonds cancel the third dipole. Hence, its overall dipole moment is 0.
-BeClBr will be linear and polar because the two substituents are not same
-In $C{H_2}C{l_2}$ , chlorine is more electronegative than Carbon which in turn is more electronegative than hydrogen, so the dipole moment will be on the right side. Same thing will happen in a vertical direction. The net dipole moment will be in the upward direction. Since, we know that the dipole moment is the vector quantity, so in north east direction, we get net dipole moment of ${2^{\frac{1}{2}}}$ times the dipole moment in horizontal or vertical direction.
-Lastly, COS has a dipole moment as it is linear but asymmetric and because of it the dipole moment does not cancel completely. The oxygen atom being more electronegative attracts the electron from the Carbon atom as compared to S, thus giving a dipole moment of 0.65 D.
Therefore, the correct answer is option (A).
Note:
Dipole moment occurs when there is separation of positive and negative charges due to the unequal attraction that the two atoms have for the bonded electrons.
Complete step by step answer:
-In $B{F_3}$ (Boron trifluoride), its shape is trigonal planar, three fluorine are attached to the boron. It has no lone pairs of electrons on B. The three F are attached to B that the resultant dipole due to two \[B - F\] bonds cancel the third dipole. Hence, its overall dipole moment is 0.

-BeClBr will be linear and polar because the two substituents are not same
-In $C{H_2}C{l_2}$ , chlorine is more electronegative than Carbon which in turn is more electronegative than hydrogen, so the dipole moment will be on the right side. Same thing will happen in a vertical direction. The net dipole moment will be in the upward direction. Since, we know that the dipole moment is the vector quantity, so in north east direction, we get net dipole moment of ${2^{\frac{1}{2}}}$ times the dipole moment in horizontal or vertical direction.

-Lastly, COS has a dipole moment as it is linear but asymmetric and because of it the dipole moment does not cancel completely. The oxygen atom being more electronegative attracts the electron from the Carbon atom as compared to S, thus giving a dipole moment of 0.65 D.
Therefore, the correct answer is option (A).
Note:
Dipole moment occurs when there is separation of positive and negative charges due to the unequal attraction that the two atoms have for the bonded electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




