
Which one of the following has the coordinate bond?
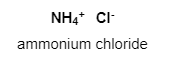
a.) ${ NH }_{ 4 }{ Cl }$
b.) ${ AlCl }_{ 3 }$
c.) ${ NaCl }$
d.) ${ Cl }_{ 2 }$
Answer
601.8k+ views
Hint: A coordinate bond is a type of covalent bond which is formed between two species in which a shared pair of electrons are donated by one species only but shared by two species.
Complete step-by-step answer:
A coordinate bond is also known as a dative bond.
Firstly, in ${ NH }_{ 4 }{ Cl }$, nitrogen has a lone pair of electrons that are responsible for the formation of coordinate covalent bonds.
Here, three covalent bonds are present between nitrogen and hydrogen, and one coordinate bond is formed between nitrogen and hydrogen.
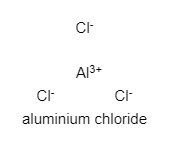
In ${ AlCl }_{ 3 }$, ionic bonds are present between aluminium and chlorine. Two of these molecules join together to form a dimer.
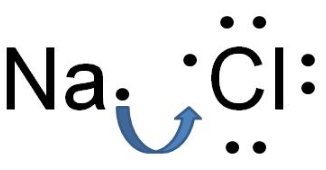
${ NaCl }$ is an ionic compound that is formed by the transfer of electrons which create ions. Here, the sodium ion is positively charged and the chloride ion is negatively charged.
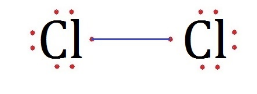
In ${ Cl }_{ 2 }$, covalent bonds are present in between Cl atoms. As both atoms share and hold tightly onto each other’s electrons.
Hence, we can say that a coordinate bond is present in ${ NH }_{ 4 }{ Cl }$.
The correct option is A.
Note: The possibility to make a mistake is that you may choose option D. ${ Cl }_{ 2 }$ has only a covalent bond between its atoms and it joins together to form a dimer.
Complete step-by-step answer:
A coordinate bond is also known as a dative bond.
Firstly, in ${ NH }_{ 4 }{ Cl }$, nitrogen has a lone pair of electrons that are responsible for the formation of coordinate covalent bonds.
Here, three covalent bonds are present between nitrogen and hydrogen, and one coordinate bond is formed between nitrogen and hydrogen.

In ${ AlCl }_{ 3 }$, ionic bonds are present between aluminium and chlorine. Two of these molecules join together to form a dimer.

${ NaCl }$ is an ionic compound that is formed by the transfer of electrons which create ions. Here, the sodium ion is positively charged and the chloride ion is negatively charged.

In ${ Cl }_{ 2 }$, covalent bonds are present in between Cl atoms. As both atoms share and hold tightly onto each other’s electrons.

Hence, we can say that a coordinate bond is present in ${ NH }_{ 4 }{ Cl }$.
The correct option is A.
Note: The possibility to make a mistake is that you may choose option D. ${ Cl }_{ 2 }$ has only a covalent bond between its atoms and it joins together to form a dimer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




