
Which one of the following has ${\text{s}}{{\text{p}}^{\text{3}}}$ , ${\text{s}}{{\text{p}}^2}$ , sp hybrid orbitals in the ratio $6:3:2$ ?
A.
B.
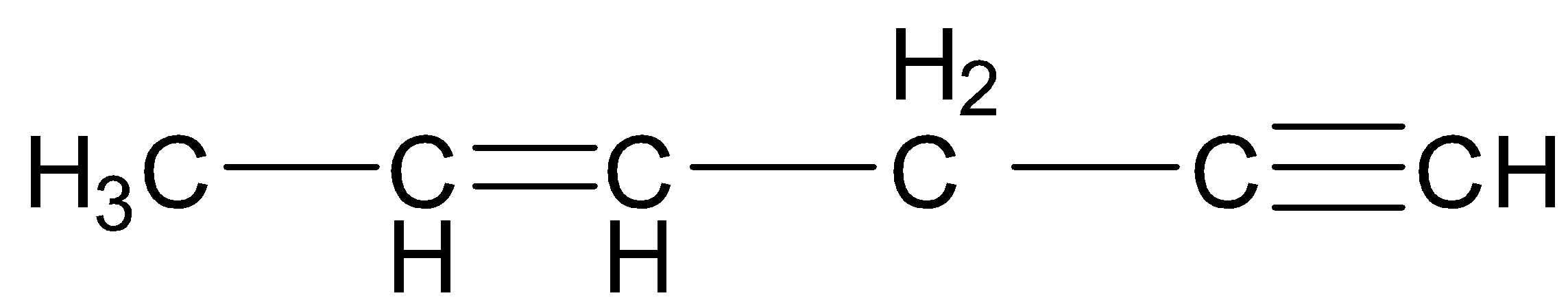
C.
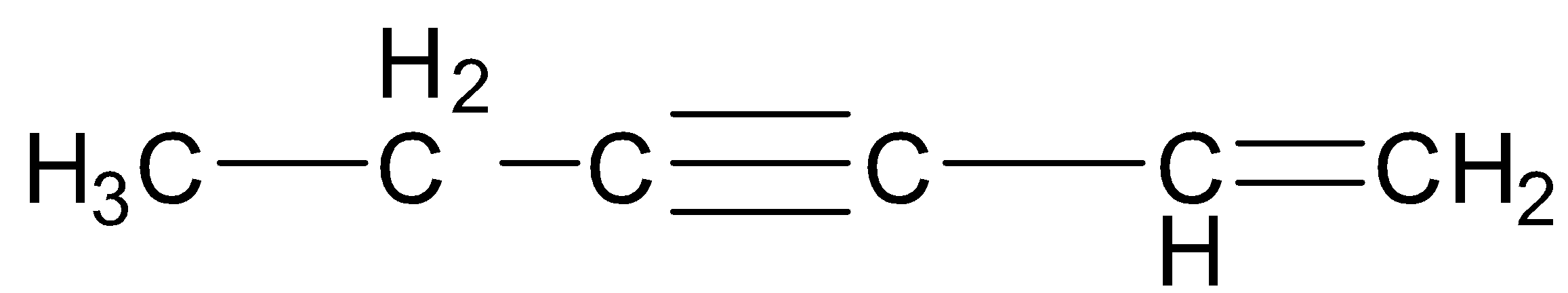
D.
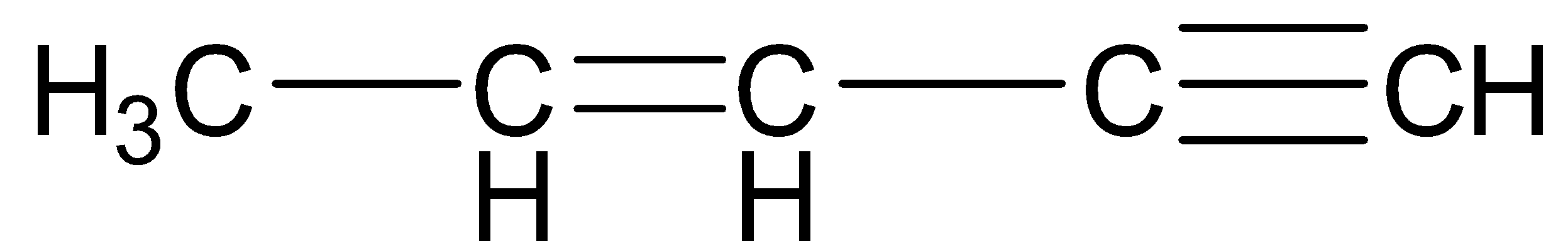


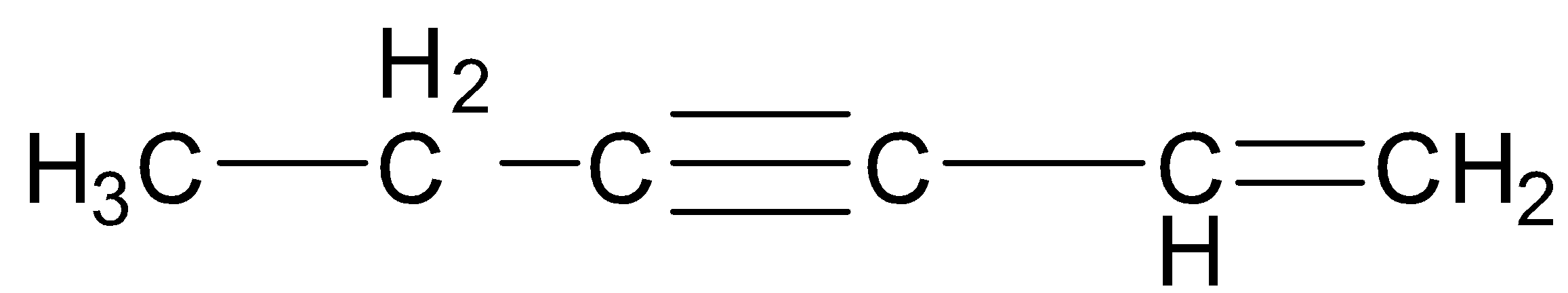

Answer
579k+ views
Hint: Hybridization refers to the phenomenon of intermixing of two or more atomic orbitals which are of nearly the same energies to give rise to the formation of new orbitals called hybrid orbitals.
For the carbon atom, hybridization takes place in three ways: ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridization, ${\text{s}}{{\text{p}}^2}$ hybridization and sp hybridization.
Each ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized carbon atom has 4 ${\text{s}}{{\text{p}}^{\text{3}}}$ hybrid orbitals, each ${\text{s}}{{\text{p}}^2}$ hybridized carbon atom has 3 ${\text{s}}{{\text{p}}^2}$ hybrid orbitals and each sp hybridized carbon atom has 2 sp hybrid orbitals.
Complete step by step answer:
A carbon atom which is bonded to other atoms by sigma bonds only is always ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized. A carbon atom which makes 3 sigma bonds and one pi bond with other atoms is always ${\text{s}}{{\text{p}}^2}$ hybridized. And lastly, a carbon atom which makes 2 sigma bonds and 2 pi bonds with other atoms is always sp hybridized.
A sigma bond can be formed independently between two atoms but a pi bond can be formed only when a sigma bond has already been formed between two atoms. This means each single bond has one sigma bond. Each double bond will also have one sigma bond. But in a double bond, a pi bond will also be present in addition to the sigma bond. Similarly, each triple bond will have a sigma bond and two pi bonds.
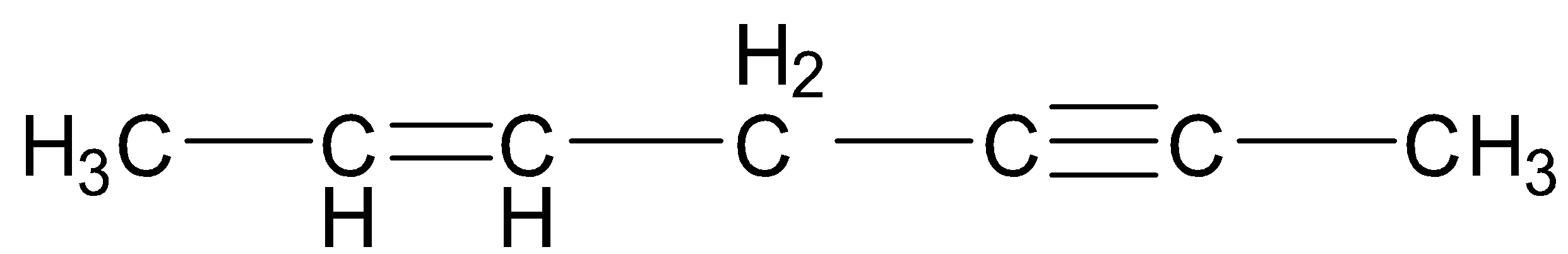
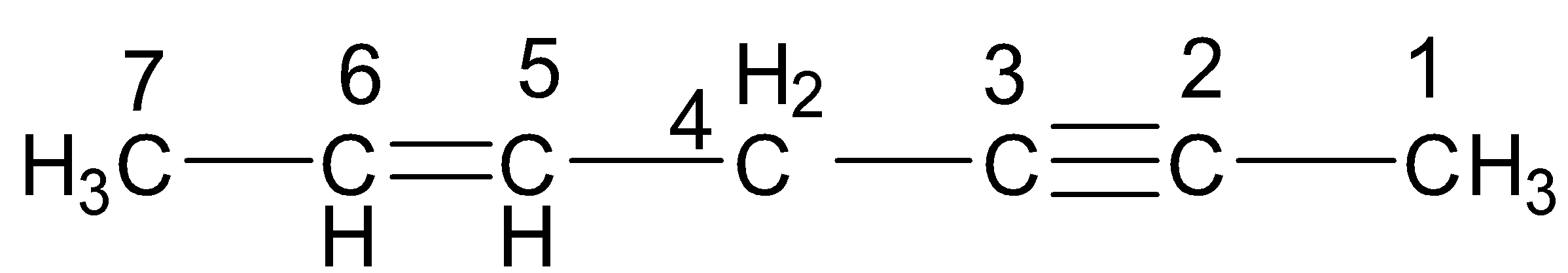
Keeping all the above facts in mind, we see that in the first compound, C-1, C-4 and C-7 are ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized, C-5 and C-6 are ${\text{s}}{{\text{p}}^2}$ hybridized and C-2 and C-3 are sp hybridized. Thus, there are a total of 3 ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized, 2 ${\text{s}}{{\text{p}}^2}$ hybridized and 2 sp hybridized carbon atoms.
Since each ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized carbon atom has 4 ${\text{s}}{{\text{p}}^{\text{3}}}$ hybrid orbitals, each ${\text{s}}{{\text{p}}^2}$ hybridized carbon atom has 3 ${\text{s}}{{\text{p}}^2}$ hybrid orbitals and each sp hybridized carbon atom has 2 sp hybrid orbitals, therefore, there are a total of 12 ${\text{s}}{{\text{p}}^{\text{3}}}$ hybrid, 6 ${\text{s}}{{\text{p}}^2}$ hybrid and 4 sp hybrid orbitals.
Hence, the required ratio is \[12:6:4\] which reduces to $6:3:2$ . This is only in the case of the first compound.
Thus, option A is correct.
Note:
With the increase in s-character, the electronegativity of the atom involved in hybridization also increases.
However, with the increase in s-character, the size of the hybrid orbitals decreases. Thus, ${\text{s}}{{\text{p}}^{\text{3}}}$ hybrid orbitals are greater in size than ${\text{s}}{{\text{p}}^2}$ hybrid orbitals which in turn are greater in size than sp hybrid orbitals.
With the increase in s-character, the bond length also decreases.
For the carbon atom, hybridization takes place in three ways: ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridization, ${\text{s}}{{\text{p}}^2}$ hybridization and sp hybridization.
Each ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized carbon atom has 4 ${\text{s}}{{\text{p}}^{\text{3}}}$ hybrid orbitals, each ${\text{s}}{{\text{p}}^2}$ hybridized carbon atom has 3 ${\text{s}}{{\text{p}}^2}$ hybrid orbitals and each sp hybridized carbon atom has 2 sp hybrid orbitals.
Complete step by step answer:
A carbon atom which is bonded to other atoms by sigma bonds only is always ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized. A carbon atom which makes 3 sigma bonds and one pi bond with other atoms is always ${\text{s}}{{\text{p}}^2}$ hybridized. And lastly, a carbon atom which makes 2 sigma bonds and 2 pi bonds with other atoms is always sp hybridized.
A sigma bond can be formed independently between two atoms but a pi bond can be formed only when a sigma bond has already been formed between two atoms. This means each single bond has one sigma bond. Each double bond will also have one sigma bond. But in a double bond, a pi bond will also be present in addition to the sigma bond. Similarly, each triple bond will have a sigma bond and two pi bonds.
Keeping all the above facts in mind, we see that in the first compound, C-1, C-4 and C-7 are ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized, C-5 and C-6 are ${\text{s}}{{\text{p}}^2}$ hybridized and C-2 and C-3 are sp hybridized. Thus, there are a total of 3 ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized, 2 ${\text{s}}{{\text{p}}^2}$ hybridized and 2 sp hybridized carbon atoms.

Since each ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridized carbon atom has 4 ${\text{s}}{{\text{p}}^{\text{3}}}$ hybrid orbitals, each ${\text{s}}{{\text{p}}^2}$ hybridized carbon atom has 3 ${\text{s}}{{\text{p}}^2}$ hybrid orbitals and each sp hybridized carbon atom has 2 sp hybrid orbitals, therefore, there are a total of 12 ${\text{s}}{{\text{p}}^{\text{3}}}$ hybrid, 6 ${\text{s}}{{\text{p}}^2}$ hybrid and 4 sp hybrid orbitals.
Hence, the required ratio is \[12:6:4\] which reduces to $6:3:2$ . This is only in the case of the first compound.
Thus, option A is correct.
Note:
With the increase in s-character, the electronegativity of the atom involved in hybridization also increases.
However, with the increase in s-character, the size of the hybrid orbitals decreases. Thus, ${\text{s}}{{\text{p}}^{\text{3}}}$ hybrid orbitals are greater in size than ${\text{s}}{{\text{p}}^2}$ hybrid orbitals which in turn are greater in size than sp hybrid orbitals.
With the increase in s-character, the bond length also decreases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




