
Which one of the following has (have) octahedral geometry?
i) $ SbC{l_6}^ - $
ii) $ SnC{l_6}^{2 - } $
iii) $ Xe{F_6} $
iv) $ IO_6^{5 - } $
A) i), ii) and iv)
B) i), iii) and iv)
C) i), ii) and iii)
D) All of these
Answer
479.1k+ views
Hint: The geometry of the molecule can be described using its hybridisation. Hybridisation is the process of combining atomic orbitals to form new hybrid atomic orbitals. These hybrid orbitals should be appropriate for electron pairing and forming of new bonds according to the Valence Bond Theory.
Complete answer:
We are given 4 compounds, all of which are polyatomic molecules/ ions. The hybridisation of polyatomic compounds can be given by the formula:
$ Hybridisation = \dfrac{{no.of{\text{ }}valence{\text{ }}electron\operatorname{s} {\text{ }}in{\text{ }}the{\text{ }}central{\text{ }}atom + no.ofHydrogen{\text{ }}Atoms + no.of{\text{ }}Halide{\text{ }}Atoms \pm Formal{\text{ }}Ch\arg e}}{2} $
If the answer obtained is:
Let us consider the given compounds one by one.
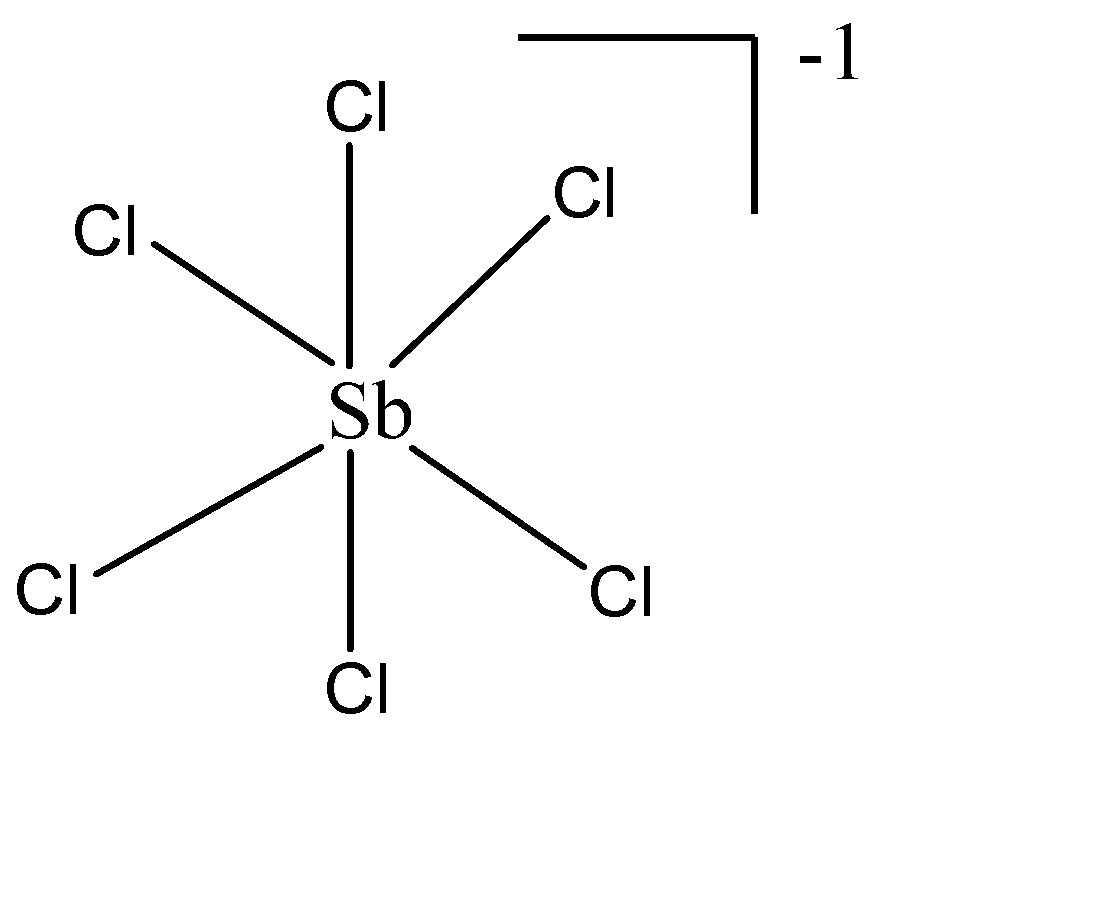
i) $ SbC{l_6}^ - $ : The central atom is Sb which belongs to group 15 of the periodic table. The no. of valence electrons are 5. The hybridisation as by the formula will be $ = \dfrac{{5 + 6 + 1}}{2} = \dfrac{{12}}{2} = 6 \to s{p^3}{d^2} $ . Therefore, the geometry would be Octahedral with zero lone pairs. The structure of the molecule will be:
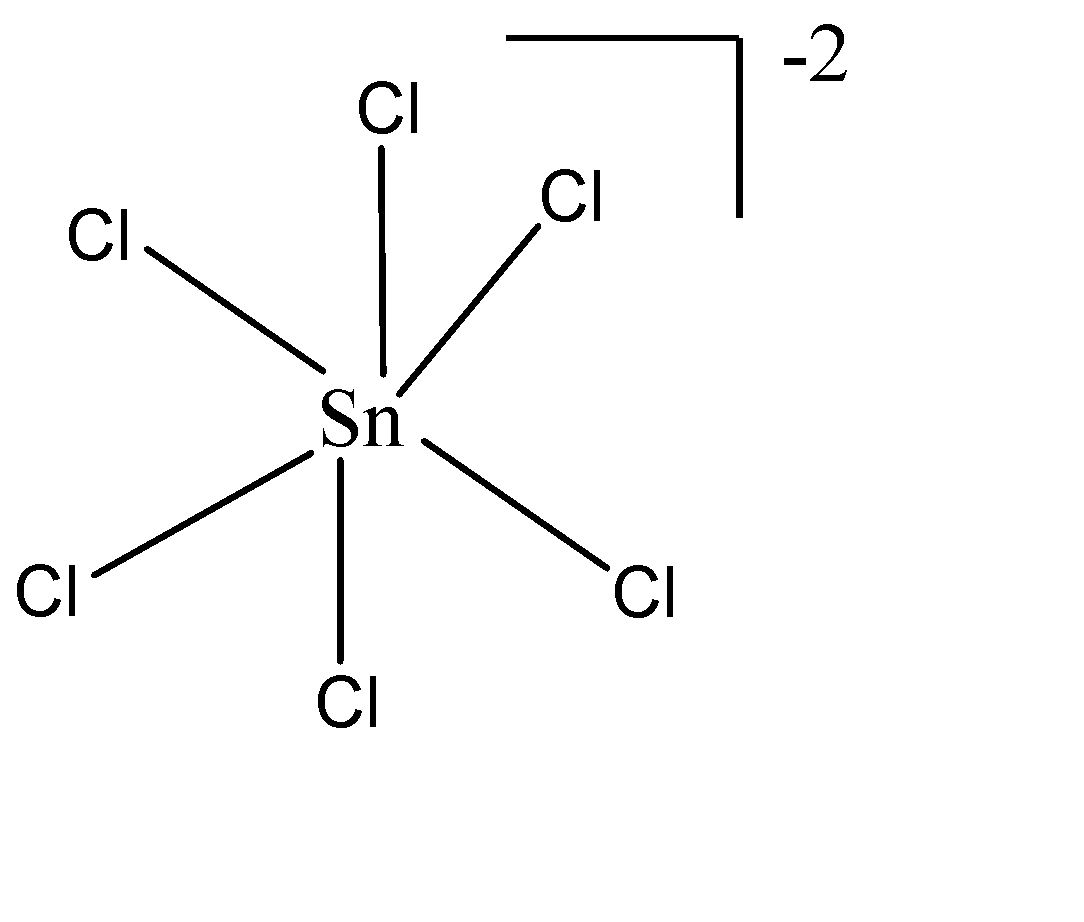
ii) $ SnC{l_6}^{2 - } $ : The central atom is Sn which belongs to group 14 of the periodic table. The no. of valence electrons is 4. The hybridisation as by the formula will be $ = \dfrac{{4 + 6 + 2}}{2} = \dfrac{{12}}{2} = 6 \to s{p^3}{d^2} $ . Therefore, the geometry would be Octahedral with zero lone pairs. The structure of the molecule will be:
iii) $ Xe{F_6} $ : The central atom is Xe which belongs to group 18 of the periodic table. The no. of valence electrons is 8. The hybridisation as by the formula will be $ = \dfrac{{8 + 6}}{2} = \dfrac{{14}}{2} = 7 \to s{p^3}{d^3} $ . Therefore, the geometry would be a distorted octahedral with one lone pair present.
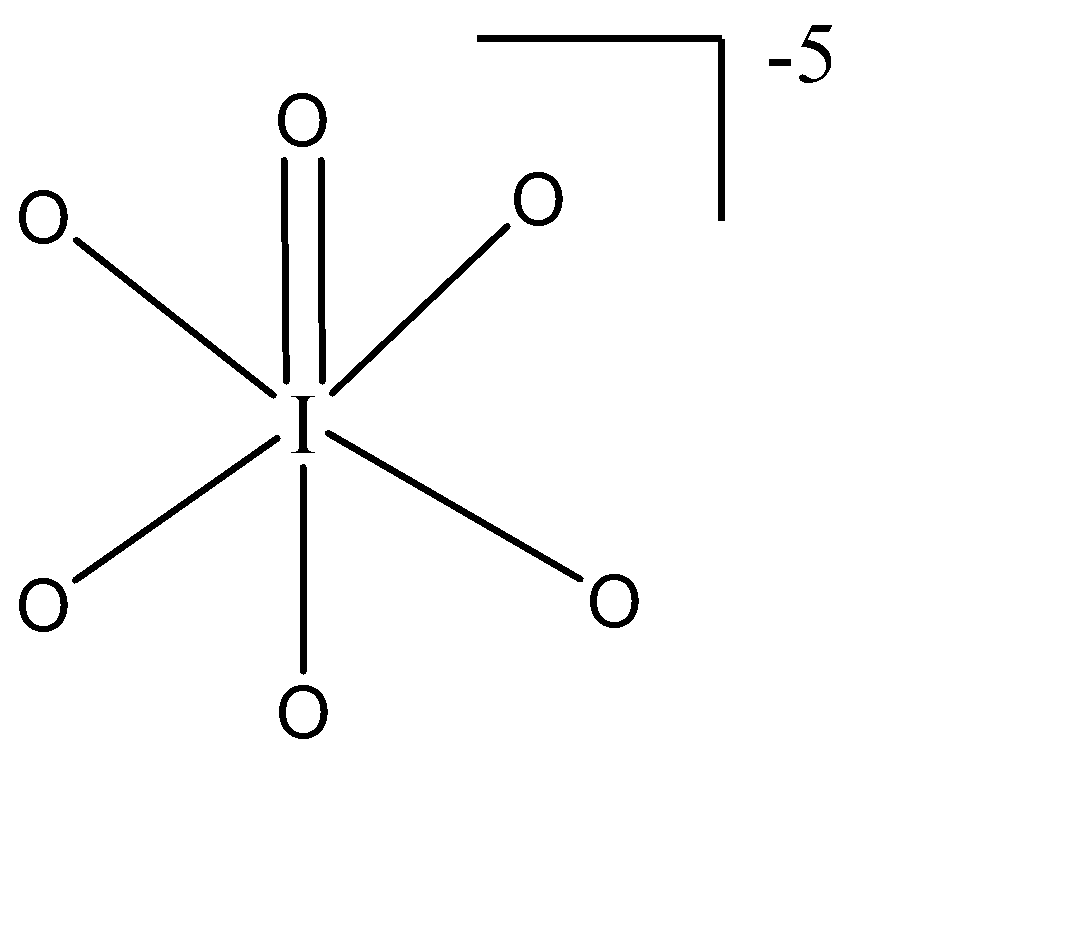
iv) $ IO_6^{5 - } $ : The central atom is Iodine which belongs to group 17 of the periodic table. The no. of valence electrons is 7. The hybridisation as by the formula will be $ = \dfrac{{7 + 5}}{2} = \dfrac{{12}}{2} = 6 \to s{p^3}{d^2} $ . Therefore, the geometry would be octahedral with zero lone pairs present.
i), ii) and iv) have octahedral geometry.
The correct option is (A).
Note:
The bond order and other properties of Diatomic species can be found out with the Help of Molecular Orbital Diagrams (MODs). MODs for polyatomic species are found to be complicated to understand. If there are lone pairs present on the central atom, the Hybridisation and geometry of the molecule will remain the same, their shape will change accordingly. For example, the shape of a molecule having Tetrahedral geometry with one L.P will be Pyramidal shaped. Similarly, molecules with Trigonal Planar geometry and one L.P will have angular shape.
Complete answer:
We are given 4 compounds, all of which are polyatomic molecules/ ions. The hybridisation of polyatomic compounds can be given by the formula:
$ Hybridisation = \dfrac{{no.of{\text{ }}valence{\text{ }}electron\operatorname{s} {\text{ }}in{\text{ }}the{\text{ }}central{\text{ }}atom + no.ofHydrogen{\text{ }}Atoms + no.of{\text{ }}Halide{\text{ }}Atoms \pm Formal{\text{ }}Ch\arg e}}{2} $
If the answer obtained is:
| Answer Obtained/Steric Number | Hybridisation | Geometry |
| 2 | sp | Linear |
| 3 | $ s{p^2} $ | Trigonal Planar |
| 4 | $ s{p^3} $ | Tetrahedral |
| 5 | $ s{p^3}d $ | Trigonal Bipyramidal |
| 6 | $ s{p^3}{d^2} $ | Octahedral |
Let us consider the given compounds one by one.
i) $ SbC{l_6}^ - $ : The central atom is Sb which belongs to group 15 of the periodic table. The no. of valence electrons are 5. The hybridisation as by the formula will be $ = \dfrac{{5 + 6 + 1}}{2} = \dfrac{{12}}{2} = 6 \to s{p^3}{d^2} $ . Therefore, the geometry would be Octahedral with zero lone pairs. The structure of the molecule will be:

ii) $ SnC{l_6}^{2 - } $ : The central atom is Sn which belongs to group 14 of the periodic table. The no. of valence electrons is 4. The hybridisation as by the formula will be $ = \dfrac{{4 + 6 + 2}}{2} = \dfrac{{12}}{2} = 6 \to s{p^3}{d^2} $ . Therefore, the geometry would be Octahedral with zero lone pairs. The structure of the molecule will be:

iii) $ Xe{F_6} $ : The central atom is Xe which belongs to group 18 of the periodic table. The no. of valence electrons is 8. The hybridisation as by the formula will be $ = \dfrac{{8 + 6}}{2} = \dfrac{{14}}{2} = 7 \to s{p^3}{d^3} $ . Therefore, the geometry would be a distorted octahedral with one lone pair present.
iv) $ IO_6^{5 - } $ : The central atom is Iodine which belongs to group 17 of the periodic table. The no. of valence electrons is 7. The hybridisation as by the formula will be $ = \dfrac{{7 + 5}}{2} = \dfrac{{12}}{2} = 6 \to s{p^3}{d^2} $ . Therefore, the geometry would be octahedral with zero lone pairs present.

i), ii) and iv) have octahedral geometry.
The correct option is (A).
Note:
The bond order and other properties of Diatomic species can be found out with the Help of Molecular Orbital Diagrams (MODs). MODs for polyatomic species are found to be complicated to understand. If there are lone pairs present on the central atom, the Hybridisation and geometry of the molecule will remain the same, their shape will change accordingly. For example, the shape of a molecule having Tetrahedral geometry with one L.P will be Pyramidal shaped. Similarly, molecules with Trigonal Planar geometry and one L.P will have angular shape.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




