
Which one of the following has a linear structure?
A) \[{\text{I}}_{\text{3}}^ - \]
B) \[{\text{N}}{{\text{O}}_2}\]
C) \[{\text{I}}_{\text{3}}^ + \]
D) \[{\text{S}}{{\text{O}}_2}\]
Answer
561k+ views
Hint: To determine the geometry and shape of the molecules one of the methods used is to draw the Lewis structure of the molecule. Then count all total electron pairs that are paired electron pairs and unpaired electron pairs. Then by using the VSEPR theory determine the electron geometry and molecular geometry of the compound.
Complete step-by-step answer:
Here, first, determine the Lewis structure of each molecule and then using the VSEPR theory determines the shape of the molecule.
Here, option(A)\[{\text{I}}_{\text{3}}^ - \].Here, the total electrons are 22. As all atoms are of iodine, the central atom is iodine. Place all these electrons around the iodine atoms as complete their valency by forming bonds between the atoms therefore, the Lewis structure of \[{\text{I}}_{\text{3}}^ - \] is as follows:
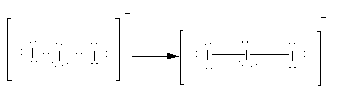
Here, there are two bond pairs and three lone pairs on the central atom. The electron pair geometry is trigonal planar while molecular geometry is linear. Therefore, option(A) \[{\text{I}}_{\text{3}}^ - \] is the correct answer to the question.
Here, option(B)\[{\text{N}}{{\text{O}}_2}\]. Here, the total electrons are 17. Here, nitrogen is central and two oxygen atoms are around it. Place all these 17 electrons around atoms and complete their valency by forming bonds between the atoms therefore, the Lewis structure of \[{\text{N}}{{\text{O}}_2}\] is as follows:
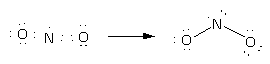
Here, there are two bond pairs and one lone pair on the central atom. The electron pair geometry is trigonal planar while molecular geometry is bent or angular.
Therefore, option(B) \[{\text{N}}{{\text{O}}_2}\] is the incorrect answer to the question.
Here, option(C)\[{\text{I}}_{\text{3}}^ + \].Here, the total electrons are 20. As all atoms are of iodine, the central atom is iodine. Place all these electrons around the iodine atoms as complete their valency by forming bonds between the atoms therefore, the Lewis structure of \[{\text{I}}_{\text{3}}^ + \] is as follows:
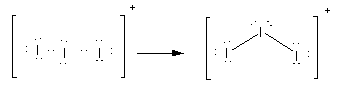
Here, there are two bond pairs and two lone pairs on the central atom. The electron pair geometry is tetrahedral while molecular geometry is angular or bent.
Therefore, option(C) \[{\text{I}}_{\text{3}}^ + \] is the incorrect answer to the question.
Here, option(D)\[{\text{S}}{{\text{O}}_2}\].Here, the total electrons are 18. Here, sulfur is central and two oxygen atoms are around it. Place all these 18 electrons around atoms and complete their valency by forming bonds between the atoms therefore, the Lewis structure of \[{\text{S}}{{\text{O}}_2}\] is as follows:

Here, there are two bond pairs and one-two lone pairs on the central atom. The electron pair geometry is trigonal planar while molecular geometry is bent or angular.
Therefore, option(B) \[{\text{S}}{{\text{O}}_2}\] is the incorrect answer to the question.
Note:Steps used to draw the Lewis structures are as follows:
1. Count the total valence electrons of all atoms of the molecules.
2. Place most electronegative atoms at the center and all other atoms surround it.
3. Place all electrons around these atoms to complete the octet of each atom.
4. If any atom fails to complete its octet then form a double or triple bond to complete the octet of the atoms.
Complete step-by-step answer:
Here, first, determine the Lewis structure of each molecule and then using the VSEPR theory determines the shape of the molecule.
Here, option(A)\[{\text{I}}_{\text{3}}^ - \].Here, the total electrons are 22. As all atoms are of iodine, the central atom is iodine. Place all these electrons around the iodine atoms as complete their valency by forming bonds between the atoms therefore, the Lewis structure of \[{\text{I}}_{\text{3}}^ - \] is as follows:

Here, there are two bond pairs and three lone pairs on the central atom. The electron pair geometry is trigonal planar while molecular geometry is linear. Therefore, option(A) \[{\text{I}}_{\text{3}}^ - \] is the correct answer to the question.
Here, option(B)\[{\text{N}}{{\text{O}}_2}\]. Here, the total electrons are 17. Here, nitrogen is central and two oxygen atoms are around it. Place all these 17 electrons around atoms and complete their valency by forming bonds between the atoms therefore, the Lewis structure of \[{\text{N}}{{\text{O}}_2}\] is as follows:

Here, there are two bond pairs and one lone pair on the central atom. The electron pair geometry is trigonal planar while molecular geometry is bent or angular.
Therefore, option(B) \[{\text{N}}{{\text{O}}_2}\] is the incorrect answer to the question.
Here, option(C)\[{\text{I}}_{\text{3}}^ + \].Here, the total electrons are 20. As all atoms are of iodine, the central atom is iodine. Place all these electrons around the iodine atoms as complete their valency by forming bonds between the atoms therefore, the Lewis structure of \[{\text{I}}_{\text{3}}^ + \] is as follows:

Here, there are two bond pairs and two lone pairs on the central atom. The electron pair geometry is tetrahedral while molecular geometry is angular or bent.
Therefore, option(C) \[{\text{I}}_{\text{3}}^ + \] is the incorrect answer to the question.
Here, option(D)\[{\text{S}}{{\text{O}}_2}\].Here, the total electrons are 18. Here, sulfur is central and two oxygen atoms are around it. Place all these 18 electrons around atoms and complete their valency by forming bonds between the atoms therefore, the Lewis structure of \[{\text{S}}{{\text{O}}_2}\] is as follows:

Here, there are two bond pairs and one-two lone pairs on the central atom. The electron pair geometry is trigonal planar while molecular geometry is bent or angular.
Therefore, option(B) \[{\text{S}}{{\text{O}}_2}\] is the incorrect answer to the question.
Note:Steps used to draw the Lewis structures are as follows:
1. Count the total valence electrons of all atoms of the molecules.
2. Place most electronegative atoms at the center and all other atoms surround it.
3. Place all electrons around these atoms to complete the octet of each atom.
4. If any atom fails to complete its octet then form a double or triple bond to complete the octet of the atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




