
Which one of the following compounds is not optically active?
(A) $C{{H}_{3}}C{{H}_{2}}CH(C{{H}_{3}})C{{H}_{2}}Cl$
(B) $C{{H}_{3}}C{{H}_{2}}CH{{(C{{H}_{3}})}_{2}}$
(C) $C{{H}_{3}}-CHOH-COOH$
(D) $C{{H}_{3}}-CHCl-C{{H}_{2}}Br$
Answer
530.1k+ views
Hint: Optical activity is only shown by chiral molecules. So, to solve this question we first need to know what chiral molecules are. A molecule that has a non-superimposable mirror image at any combination of translations and rotations is known as a chiral molecule.
Complete answer:
To determine which molecule is not optically active we have to determine which molecule is not chiral.
Now, in a chiral molecule, there is a presence of chiral carbon. The carbon atom that contains four different bonded groups, including lone pairs, is known as a chiral carbon.
First, let us draw the structures of each molecule.
Now, let us determine the presence of chiral atoms in each of the options
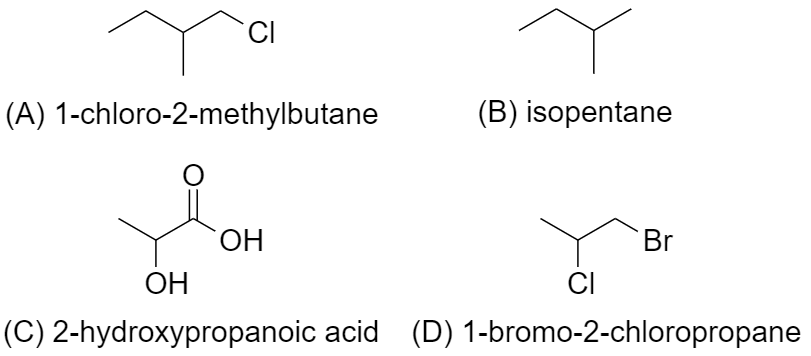
(A) In 1-Chloro-2methylbutane ($C{{H}_{3}}C{{H}_{2}}CH(C{{H}_{3}})C{{H}_{2}}Cl$), there is 1 chiral carbon.
Since it has a chiral carbon, which is attached to 4 distinct bonding group, which are $-C{{H}_{2}}C{{H}_{3}}$, $-C{{H}_{3}}$, -H, and $-C{{H}_{2}}Cl$
Hence, it is optically active.
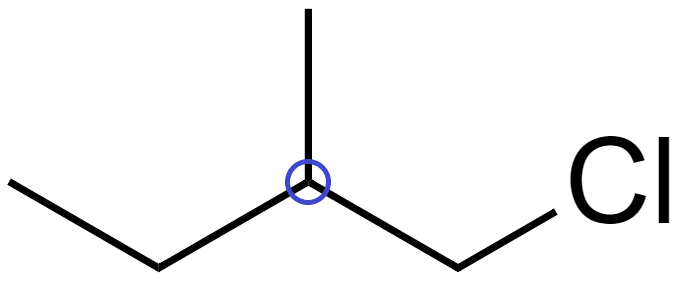
(B) In isopentane ($C{{H}_{3}}C{{H}_{2}}CH{{(C{{H}_{3}})}_{2}}$), there are no chiral carbons.
Since no atom is attached to 4 distinct bonding groups, it does not have a chiral carbon and hence is not optically active.
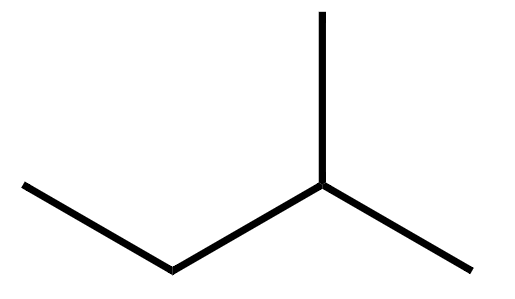
(C) In 2-hydroxypropanoic acid ($C{{H}_{3}}-CHOH-COOH$), there is 1 chiral carbon.
Since it has a chiral carbon, which is attached to 4 distinct bonding groups, which are $-COOH$, $-C{{H}_{3}}$, -OH, and -H.
Hence, it is optically active.
(D) In 1-Bromo-2-chloropropane ($C{{H}_{3}}-CHCl-C{{H}_{2}}Br$), there is 1 chiral carbon.
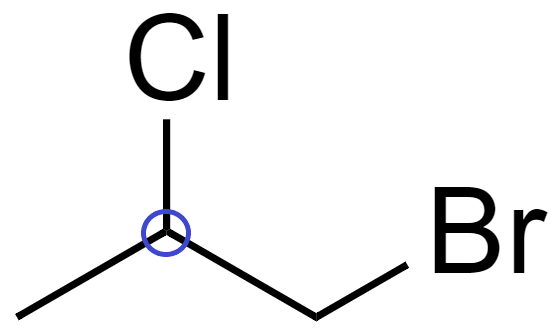
Since it has a chiral carbon, which is attached to 4 distinct bonding group which are $-C{{H}_{3}}$, -Cl, -H and $-C{{H}_{2}}Br$
Hence, it is optically active.
So, the correct option is option (B) $C{{H}_{3}}C{{H}_{2}}CH{{(C{{H}_{3}})}_{2}}$ (isopentane).
Note:
It should be noted that an atom having 2 or more than 2 identical groups attached to it is not a chiral center.
For example, the straight-chain alkyl group's carbon atoms are not chiral, as they have multiple hydrogens attached to them $(-C{{H}_{2}}\text{ or -C}{{\text{H}}_{3}})$.
A carbon atom having multiple bonding like a double bond or triple bond is not chiral as it does not have four distinct ligands.
Complete answer:
To determine which molecule is not optically active we have to determine which molecule is not chiral.
Now, in a chiral molecule, there is a presence of chiral carbon. The carbon atom that contains four different bonded groups, including lone pairs, is known as a chiral carbon.
First, let us draw the structures of each molecule.

Now, let us determine the presence of chiral atoms in each of the options
(A) In 1-Chloro-2methylbutane ($C{{H}_{3}}C{{H}_{2}}CH(C{{H}_{3}})C{{H}_{2}}Cl$), there is 1 chiral carbon.

Since it has a chiral carbon, which is attached to 4 distinct bonding group, which are $-C{{H}_{2}}C{{H}_{3}}$, $-C{{H}_{3}}$, -H, and $-C{{H}_{2}}Cl$
Hence, it is optically active.
(B) In isopentane ($C{{H}_{3}}C{{H}_{2}}CH{{(C{{H}_{3}})}_{2}}$), there are no chiral carbons.

Since no atom is attached to 4 distinct bonding groups, it does not have a chiral carbon and hence is not optically active.
(C) In 2-hydroxypropanoic acid ($C{{H}_{3}}-CHOH-COOH$), there is 1 chiral carbon.

Since it has a chiral carbon, which is attached to 4 distinct bonding groups, which are $-COOH$, $-C{{H}_{3}}$, -OH, and -H.
Hence, it is optically active.
(D) In 1-Bromo-2-chloropropane ($C{{H}_{3}}-CHCl-C{{H}_{2}}Br$), there is 1 chiral carbon.

Since it has a chiral carbon, which is attached to 4 distinct bonding group which are $-C{{H}_{3}}$, -Cl, -H and $-C{{H}_{2}}Br$
Hence, it is optically active.
So, the correct option is option (B) $C{{H}_{3}}C{{H}_{2}}CH{{(C{{H}_{3}})}_{2}}$ (isopentane).
Note:
It should be noted that an atom having 2 or more than 2 identical groups attached to it is not a chiral center.
For example, the straight-chain alkyl group's carbon atoms are not chiral, as they have multiple hydrogens attached to them $(-C{{H}_{2}}\text{ or -C}{{\text{H}}_{3}})$.
A carbon atom having multiple bonding like a double bond or triple bond is not chiral as it does not have four distinct ligands.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




