
Which one of the following alkenes will react faster with $H_2$ under catalytic hydrogenation conditions?
A.
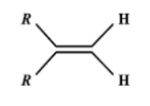
B.

C.
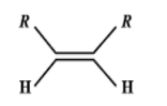
D.
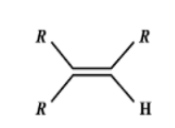




Answer
580.8k+ views
Hint : Students must know that stability of alkene is inversely proportional to the heat of hydrogenation of alkene. Therefore as the hydrogenation increases/decreases the stability of alkene will increase or decrease depending upon the process of hydrogenation.
Complete step by step answer:-
Hydrogenation is a chemical process in which molecular hydrogen reacts with compounds or elements present in the presence of catalysts such as platinum, nickel etc. During this process the hydrogen attaches with the molecule having a double or triple bond. Resulting in dissociation of molecules. During catalytic hydrogenation, The hydrogen is transferred from the catalyst to the same side of the double bond.
Evidently, small the number of R-substituents, lesser is the steric hindrance and hence faster is the rate of hydrogenation..
Catalytic hydrogenation takes place in the 3rd compound. The energy required to break the double bond is lesser in the 3rd compound than any other compound. Greater the number of alkyl groups attached to the doubly bonded carbon atoms, more stable is the alkene. (less reactive).
Thus the option (a) with two groups on the same side of the molecule is correct.
Hence (A) is the correct answer
Note :
A student may forget the inverse relation of heat of hydrogenation and stability. One may also get perplexed between the converse effect of R-substituents on the rate of hydrogenation. It is advised to take proper notes on the effect of stability on alkenes.
Complete step by step answer:-
Hydrogenation is a chemical process in which molecular hydrogen reacts with compounds or elements present in the presence of catalysts such as platinum, nickel etc. During this process the hydrogen attaches with the molecule having a double or triple bond. Resulting in dissociation of molecules. During catalytic hydrogenation, The hydrogen is transferred from the catalyst to the same side of the double bond.
Evidently, small the number of R-substituents, lesser is the steric hindrance and hence faster is the rate of hydrogenation..
Catalytic hydrogenation takes place in the 3rd compound. The energy required to break the double bond is lesser in the 3rd compound than any other compound. Greater the number of alkyl groups attached to the doubly bonded carbon atoms, more stable is the alkene. (less reactive).
Thus the option (a) with two groups on the same side of the molecule is correct.
Hence (A) is the correct answer
Note :
A student may forget the inverse relation of heat of hydrogenation and stability. One may also get perplexed between the converse effect of R-substituents on the rate of hydrogenation. It is advised to take proper notes on the effect of stability on alkenes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




