
Which one is the correct graph (figure) for the corresponding acid base titration?
A.
B.
C.
D. All of these



Answer
531.9k+ views
Hint: If an acid has a low pH (1-3) then the acid is called strong acid and the acid has high pH (4-7) then it is called weak acid. If a base has high pH (11-14) then the base is called strong base and the base has low pH (7-10) then it is called weak base.
Complete answer:
- In the question it is asked to identify which graph in the given options is suitable for acid base titration.
- We have to find the graph which is suitable for the acid-base titration.
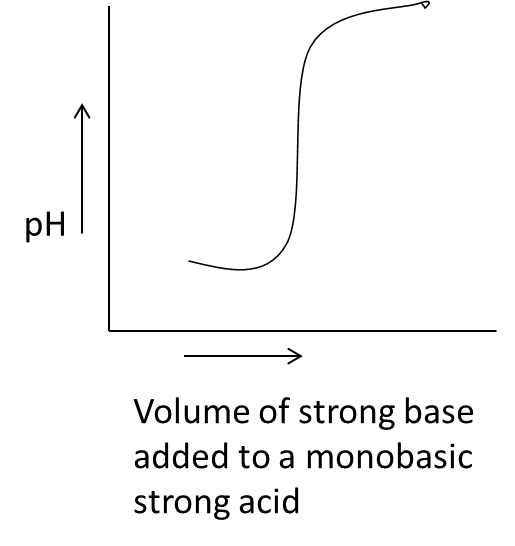
- Coming to the given options, option A.
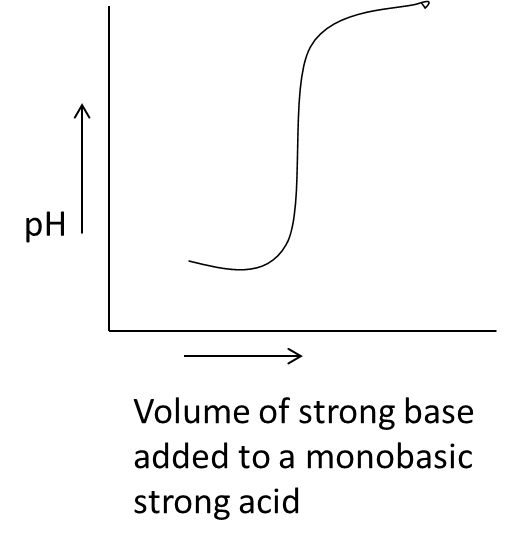
- In the above graph we can see that on the x-axis the parameter is the volume of a strong base added to a monobasic strong acid against the pH on y-axis.
- We know that by adding a small amount of strong base to a monobasic strong acid the pH of the solution is going to increase sharply and we can see the same in the graph A.
- Therefore the graph A is correct for strong acid vs strong base.
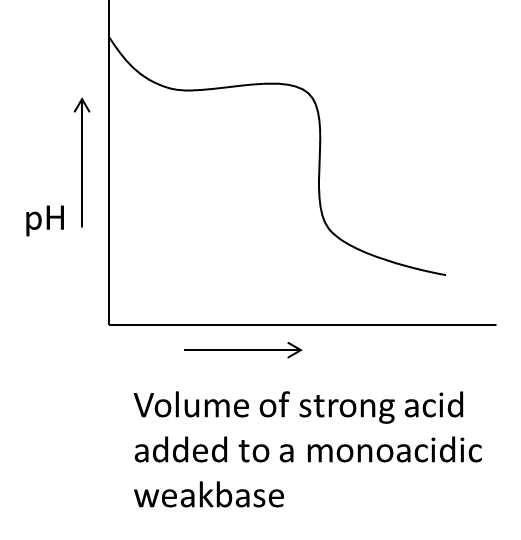
- Coming to option B,
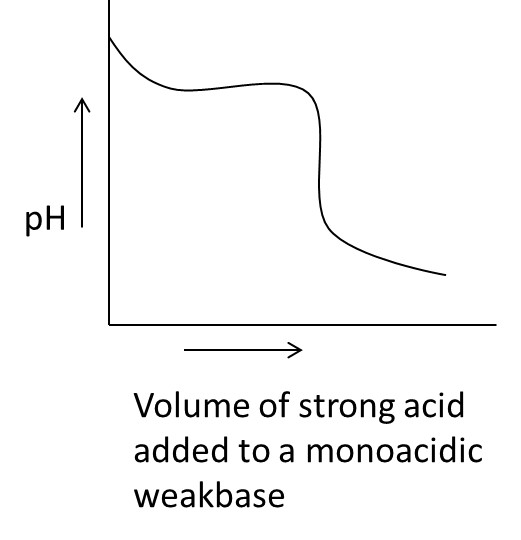
- In the above graph we can see that on the x-axis the parameter is the volume of strong acid added to a monoacidic weak base against the pH on y-axis.
- We know that when we are going to add a strong acid to monoacidic weak base initially the pH of the solution is going to decrease and it will be constant till some volume later the pH of the monoacidic weka base is going to decrease sharply due to the addition of strong acid.
- Therefore option B is also correct for acid base titration.
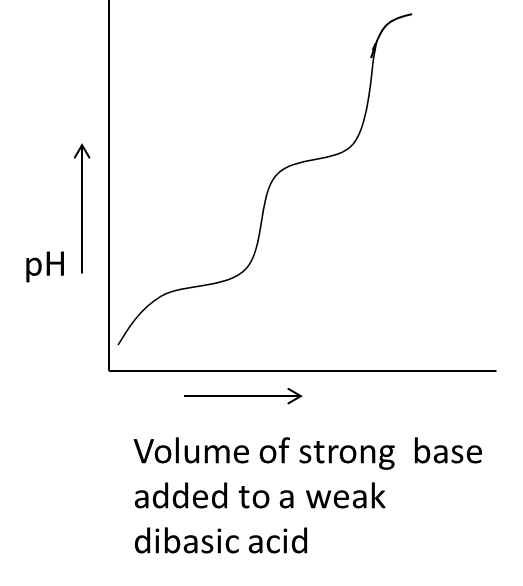
- Coming to option C,
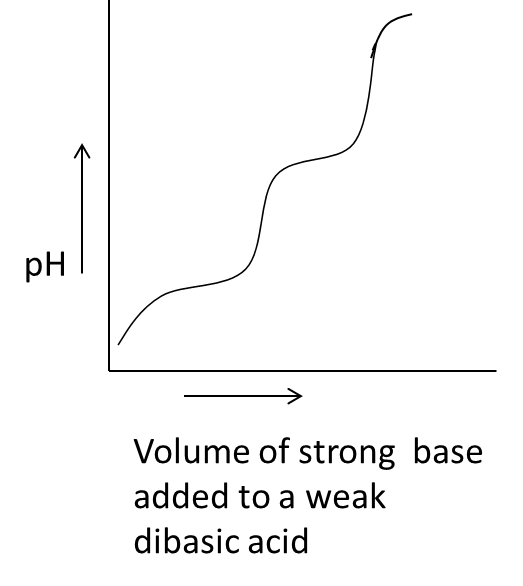
- In the above graph we can see that on the x-axis the parameter is the volume of the strong base added to a weak dibasic acid against the pH on y-axis.
- We know that whenever we are going to add a strong base to weak dibasic acid the pH of the solution increases and decreases continuously by the addition of a strong base.
- Therefore option C is also correct and related to acid-base titration.
Therefore the correct option is D, all of these.
Note:
At the time of addition of a strong acid to a base the pH of the solution is going to decrease due to the neutralization of the base with the protons which are going to be released from the strong acid. If we are going to add a base to an acid the pH of the solution is going to increase.
Complete answer:
- In the question it is asked to identify which graph in the given options is suitable for acid base titration.
- We have to find the graph which is suitable for the acid-base titration.
- Coming to the given options, option A.

- In the above graph we can see that on the x-axis the parameter is the volume of a strong base added to a monobasic strong acid against the pH on y-axis.
- We know that by adding a small amount of strong base to a monobasic strong acid the pH of the solution is going to increase sharply and we can see the same in the graph A.
- Therefore the graph A is correct for strong acid vs strong base.
- Coming to option B,

- In the above graph we can see that on the x-axis the parameter is the volume of strong acid added to a monoacidic weak base against the pH on y-axis.
- We know that when we are going to add a strong acid to monoacidic weak base initially the pH of the solution is going to decrease and it will be constant till some volume later the pH of the monoacidic weka base is going to decrease sharply due to the addition of strong acid.
- Therefore option B is also correct for acid base titration.
- Coming to option C,

- In the above graph we can see that on the x-axis the parameter is the volume of the strong base added to a weak dibasic acid against the pH on y-axis.
- We know that whenever we are going to add a strong base to weak dibasic acid the pH of the solution increases and decreases continuously by the addition of a strong base.
- Therefore option C is also correct and related to acid-base titration.
Therefore the correct option is D, all of these.
Note:
At the time of addition of a strong acid to a base the pH of the solution is going to decrease due to the neutralization of the base with the protons which are going to be released from the strong acid. If we are going to add a base to an acid the pH of the solution is going to increase.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




