
Which of the molecules is not tetrahedral?
$A.$ $C{F_4}$
$B.$ $S{F_4}$
$C.$ $C{H_4}$
$D.$ $Si{F_4}$
Answer
543.6k+ views
Hint:Four electrons pairs are distributed in a tetrahedral shape. If these are all bond pairs the molecular geometry is tetrahedral. If there is one lone pair of electrons and four bond pairs then the geometry is trigonal bipyramidal.
Complete step-by-step answer:
In the given question we have given four molecules namely $C{F_4}$ , $S{F_4}$ , $C{H_4}$ and $Si{F_4}$ . We have to find which one is not tetrahedral let check them one by one.
$C{F_4}$ Molecule has four electron pairs all pairs are bond pairs so according to the $VSEPR$ theory the shape of the molecule is tetrahedral.
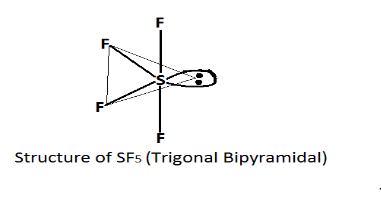
In $S{F_4}$ sulfur tetrafluoride has $5$ region of electron density around the central atom (sulfur). In which four bonds and one lone pair, According to the $VSEPR$ theory these are arranged in a trigonal bipyramidal shape.
In $C{H_4}$ molecule has four electron pairs all the pairs are bond pair so, According to $VSEPR$ theory these are arranged in a tetrahedral shape.
In the last molecule $Si{F_4}$ have four electron pairs all the pairs are bond pair so, According to $VSEPR$ theory these are arranged in a tetrahedral shape
Thus the correct answer is $S{F_4}$ as its shape is trigonal bipyramidal.
Note: Valence Shell Electron Pair Repulsion Theory $\left( {VSEPR} \right)$ .This is a very useful theory to predict the geometry or shape of a number of polyatomic molecules or ions on a non-transition element. This theory says that shapes of a species depend on the number of and nature of electron pairs surrounding the central atom of a species.
Table summarizes the relationship between the number of electron pairs that are bond pairs and lone pairs and the shape or geometry.
Complete step-by-step answer:
In the given question we have given four molecules namely $C{F_4}$ , $S{F_4}$ , $C{H_4}$ and $Si{F_4}$ . We have to find which one is not tetrahedral let check them one by one.
$C{F_4}$ Molecule has four electron pairs all pairs are bond pairs so according to the $VSEPR$ theory the shape of the molecule is tetrahedral.
In $S{F_4}$ sulfur tetrafluoride has $5$ region of electron density around the central atom (sulfur). In which four bonds and one lone pair, According to the $VSEPR$ theory these are arranged in a trigonal bipyramidal shape.
In $C{H_4}$ molecule has four electron pairs all the pairs are bond pair so, According to $VSEPR$ theory these are arranged in a tetrahedral shape.
In the last molecule $Si{F_4}$ have four electron pairs all the pairs are bond pair so, According to $VSEPR$ theory these are arranged in a tetrahedral shape
Thus the correct answer is $S{F_4}$ as its shape is trigonal bipyramidal.

Note: Valence Shell Electron Pair Repulsion Theory $\left( {VSEPR} \right)$ .This is a very useful theory to predict the geometry or shape of a number of polyatomic molecules or ions on a non-transition element. This theory says that shapes of a species depend on the number of and nature of electron pairs surrounding the central atom of a species.
Table summarizes the relationship between the number of electron pairs that are bond pairs and lone pairs and the shape or geometry.
| Total number of electron pairs | Shape |
| 2 | Linear |
| 3 | Trigonal planar |
| 4 | Tetrahedral |
| 5 | Trigonal bipyramidal |
| 6 | Octahedral |
| 7 | Pentagonal bipyramidal |
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




