
Which of the molecule or ion does not have same number of lone pairs: -
This question has multiple correct options
1. $S{F_4}$
2. $P{H_3}$
3. $ClO_3^{ - 1}$
4. $Xe{F_2}$
Answer
573.6k+ views
Hint: Lone pair is a pair of valence shell electrons that is not shared with another atom in the bond formation. It is also called a nonbonding pair or unshared pair of electrons. They are present in the outermost shell of an atom. Every atom has a different number of lone pairs. They can be identified by using Lewis structure. The number of bonding pair electrons plus the number of lone pairs is the total number of valence shell electrons of an atom.
Complete step by step answer:
1.First option is $S{F_4}$. The structure of $S{F_4}$ (sulfur tetrafluoride) molecule is:
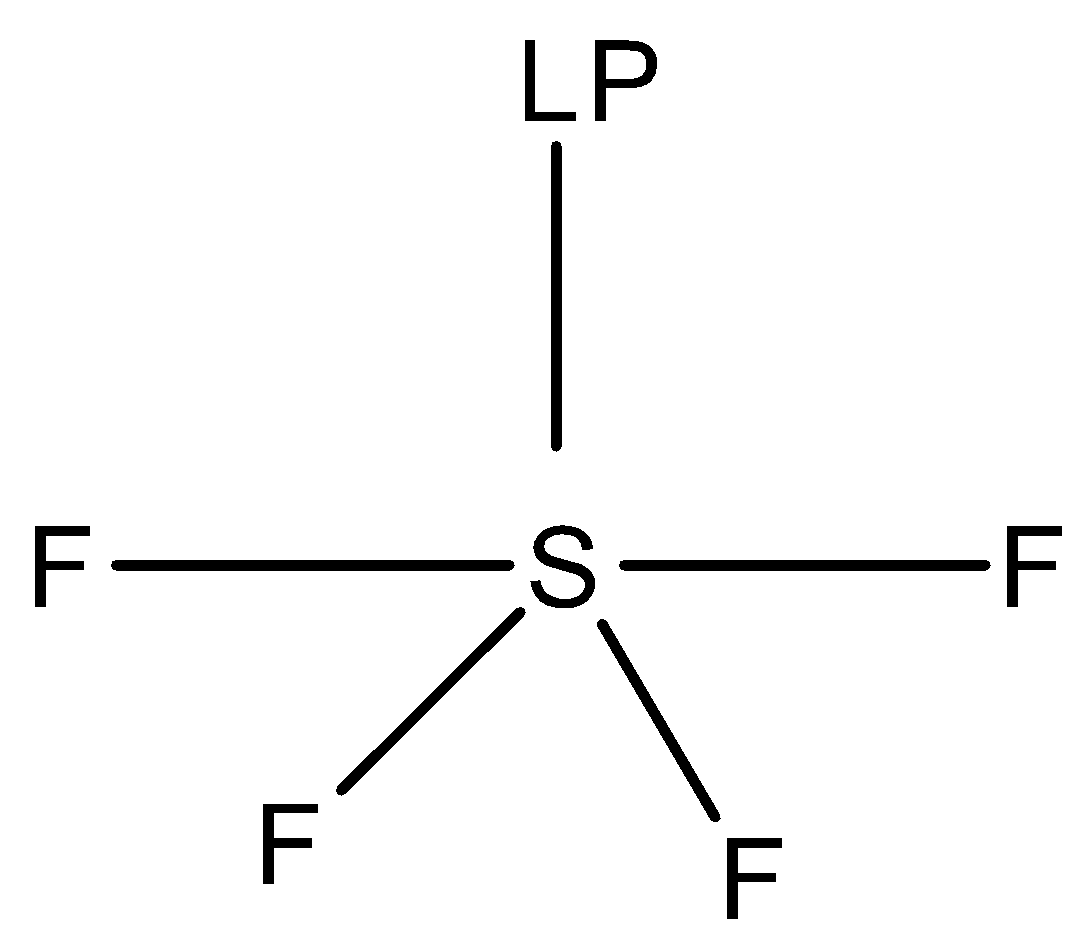
In the structure of $S{F_4}$ , we can see that the sulfur atom forms four sigma bonds with four fluorine atoms and one lone pair is present. The molecule is $s{p^3}d$ hybridized with trigonal bipyramidal geometry and the shape of the molecule is See saw.
2.Second option is $P{H_3}$. The structure of $P{H_3}$ (Phosphine) molecule is:
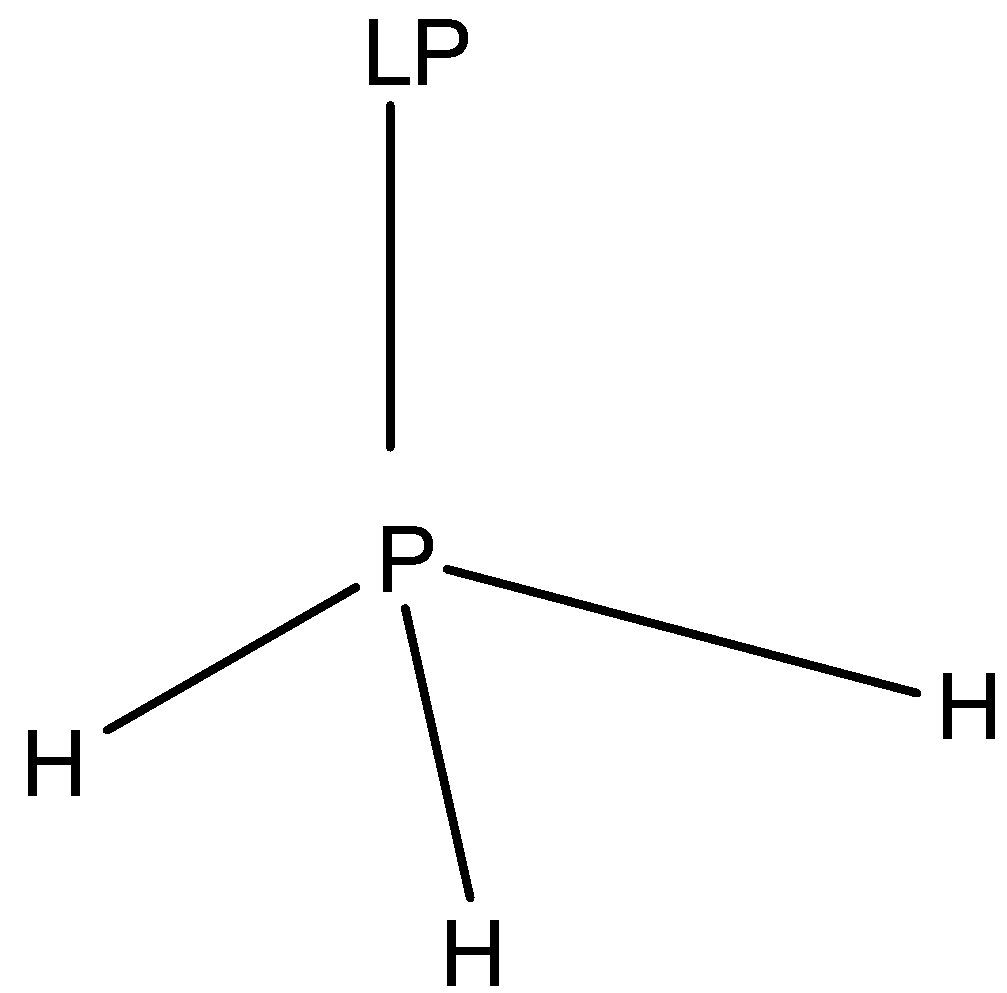
In the structure of $P{H_3}$, we can see that the phosphorus atom forms three sigma bonds with three hydrogen atoms and one lone pair is present. The molecule has a distorted tetrahedral geometry and the shape of the molecule is Pyramidal.
3.Third option is $ClO_3^{ - 1}$. The structure of $ClO_3^{ - 1}$ (chlorate ion) is:
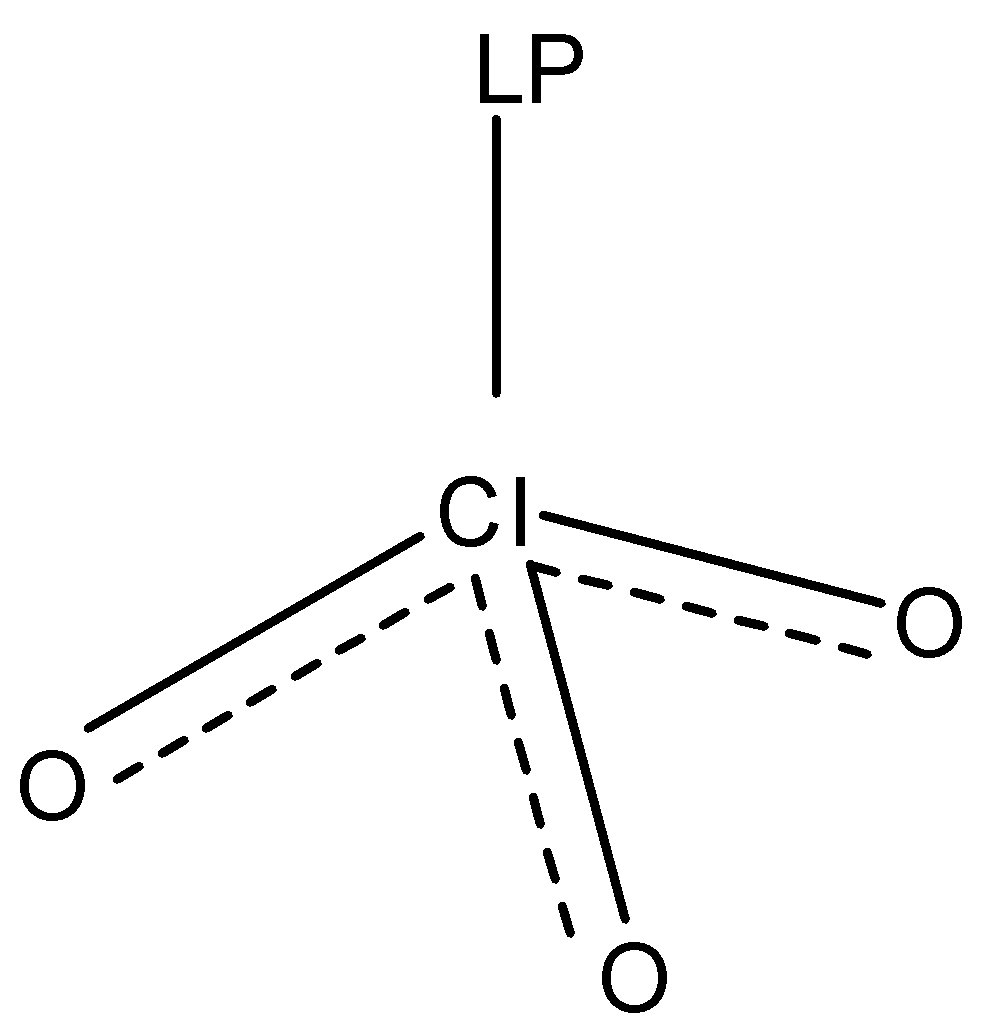
In the structure of $ClO_3^{ - 1}$, we can see that the chlorine atom forms three sigma bonds and three pi- bonds (double bonds) with three oxygen atoms and one lone pair is present. The molecule is $s{p^3}$ hybridized with a tetrahedral geometry and the shape of the molecule is Pyramidal.
4.Fourth option is $Xe{F_2}$. The structure of $Xe{F_2}$ (Xenon difluoride) is:
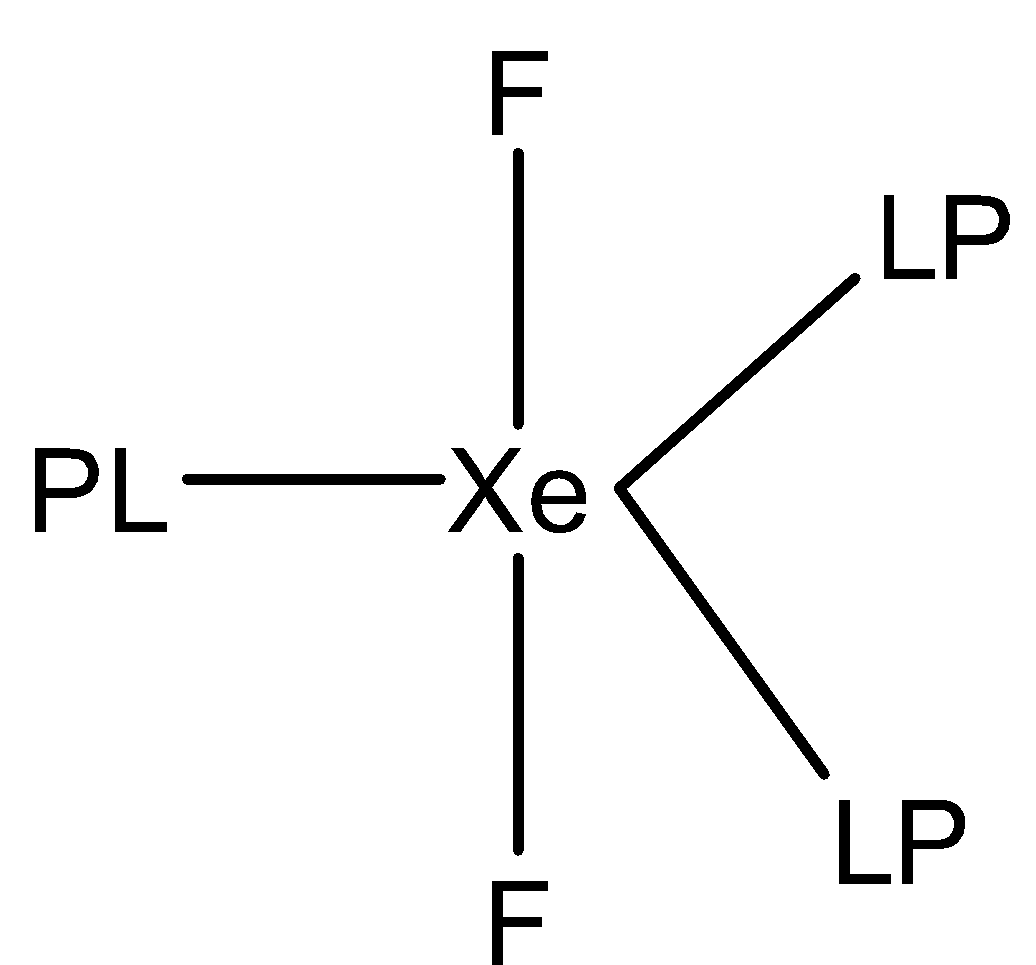
In the structure of $Xe{F_2}$, we can see that the xenon atom forms two sigma bonds with two fluorine atoms and three lone pairs are present. The molecule is $s{p^3}d$ hybridized and the shape of the molecule is linear.
After discussing we can conclude that $S{F_4}$ ,$P{H_3}$, $ClO_3^{ - 1}$ have the same number of lone pairs.
So, the correct answer is Option 1,2,3.
Note: 1.The lone pairs often exhibit negative polar characters due to their high charge density and are located closer to the nucleus of the atom.
2.The presence of a lone pair in an atom decreases the bond angle between the bonding pair of electrons due to their high charge which causes more repulsion between the electrons.
3.Lone pairs are also used in the forming of coordinate covalent bonds.
Complete step by step answer:
1.First option is $S{F_4}$. The structure of $S{F_4}$ (sulfur tetrafluoride) molecule is:

In the structure of $S{F_4}$ , we can see that the sulfur atom forms four sigma bonds with four fluorine atoms and one lone pair is present. The molecule is $s{p^3}d$ hybridized with trigonal bipyramidal geometry and the shape of the molecule is See saw.
2.Second option is $P{H_3}$. The structure of $P{H_3}$ (Phosphine) molecule is:

In the structure of $P{H_3}$, we can see that the phosphorus atom forms three sigma bonds with three hydrogen atoms and one lone pair is present. The molecule has a distorted tetrahedral geometry and the shape of the molecule is Pyramidal.
3.Third option is $ClO_3^{ - 1}$. The structure of $ClO_3^{ - 1}$ (chlorate ion) is:

In the structure of $ClO_3^{ - 1}$, we can see that the chlorine atom forms three sigma bonds and three pi- bonds (double bonds) with three oxygen atoms and one lone pair is present. The molecule is $s{p^3}$ hybridized with a tetrahedral geometry and the shape of the molecule is Pyramidal.
4.Fourth option is $Xe{F_2}$. The structure of $Xe{F_2}$ (Xenon difluoride) is:

In the structure of $Xe{F_2}$, we can see that the xenon atom forms two sigma bonds with two fluorine atoms and three lone pairs are present. The molecule is $s{p^3}d$ hybridized and the shape of the molecule is linear.
After discussing we can conclude that $S{F_4}$ ,$P{H_3}$, $ClO_3^{ - 1}$ have the same number of lone pairs.
So, the correct answer is Option 1,2,3.
Note: 1.The lone pairs often exhibit negative polar characters due to their high charge density and are located closer to the nucleus of the atom.
2.The presence of a lone pair in an atom decreases the bond angle between the bonding pair of electrons due to their high charge which causes more repulsion between the electrons.
3.Lone pairs are also used in the forming of coordinate covalent bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




