
Which of the gases diffused faster among ${N_2},{O_2},C{H_4}$ . Why?
Answer
585.3k+ views
Hint:Basically, the rate of diffusion of gases is inversely proportional to the molecular weight. The lighter the gas, the faster is the diffusion. So to find out that which gas diffused faster, we need to know the molecular weight of all the gases.
Complete step by step answer:
Diffusion is the net movement of atoms and molecules form highly concentrated region to low concentration region. Moreover, it is the movement of atoms or molecules from high chemical potential region to the low chemical potential region.
Further, the movement of molecules in solids is very small, relatively large in liquids and very large in gases and this movement of molecules leads to a phenomenon called as diffusion.
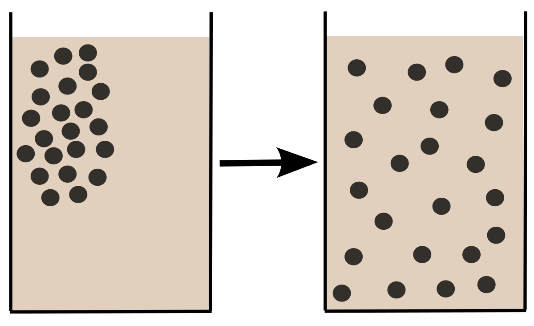
Moreover, the thermal motion of gas particles at above absolute zero temperature is known as molecular diffusion. The rate of this phenomenon movement is a function of the viscosity of the gas, size of the particles and temperature. Therefore, the result of diffusion is a slow mixing of materials where the distribution of molecules or atoms are uniform.
Now, as we know that the rate of diffusion of gases is inversely proportional to the molecular weight i.e. the lighter the gas, the faster is the diffusion. So on the basis of the molecular weight of the above gases, we can determine that which gas will diffuse faster.
The molecular weight of $C{H_4}$ is 16, ${N_2}$ is 28 and ${O_2}$ is 32. So, $C{H_4}$ will diffuse faster, followed by ${N_2}$ and ${O_2}$
Note:Diffusion should not be confused with other transport phenomena such as advection or convection. In this type of phenomenon there is movement of particles from one place to another in a bulk motion. Advection is transport with means of fluid flow whereas diffusion is the transport of compounds by the action of random motions.
Complete step by step answer:
Diffusion is the net movement of atoms and molecules form highly concentrated region to low concentration region. Moreover, it is the movement of atoms or molecules from high chemical potential region to the low chemical potential region.
Further, the movement of molecules in solids is very small, relatively large in liquids and very large in gases and this movement of molecules leads to a phenomenon called as diffusion.

Moreover, the thermal motion of gas particles at above absolute zero temperature is known as molecular diffusion. The rate of this phenomenon movement is a function of the viscosity of the gas, size of the particles and temperature. Therefore, the result of diffusion is a slow mixing of materials where the distribution of molecules or atoms are uniform.
Now, as we know that the rate of diffusion of gases is inversely proportional to the molecular weight i.e. the lighter the gas, the faster is the diffusion. So on the basis of the molecular weight of the above gases, we can determine that which gas will diffuse faster.
The molecular weight of $C{H_4}$ is 16, ${N_2}$ is 28 and ${O_2}$ is 32. So, $C{H_4}$ will diffuse faster, followed by ${N_2}$ and ${O_2}$
Note:Diffusion should not be confused with other transport phenomena such as advection or convection. In this type of phenomenon there is movement of particles from one place to another in a bulk motion. Advection is transport with means of fluid flow whereas diffusion is the transport of compounds by the action of random motions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




