
Which of the following would give Hoffmann alkene? This question has multiple correct options.
A)
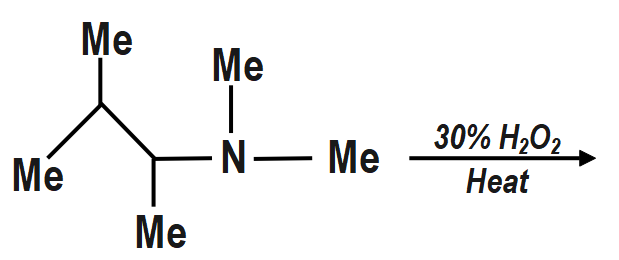
B)
C)
D)
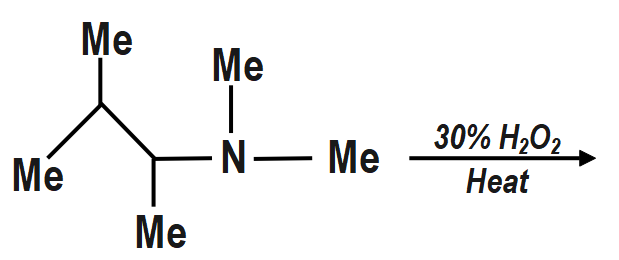



Answer
526.8k+ views
Hint: Alkenes are the one with at least one carbon-carbon double bond. They are very important for the chemical industry as they are produced due to the cracking of alkanes. They also burn in air and form carbon dioxide and water. Ethene in oxygen reacts explosively so it is not much good as a fuel. They can be used in the chemical industry too where they are used to produce plastics and many other chemicals which are to be used as fuels.
Complete step by step solution:
It does not have beta hydrogens present which is why methyl iodide is used in excess which thus cannot complete elimination. If there are two different sets of beta hydrogen present in the alkyl group then the alkene isomer having less substituted double bond is made as the major product of the reaction.
The Hofmann rule states that the major alkene product is the least substituted and least stable product when it comes to asymmetrical amines. The Hofmann elimination can be illustrated as follows:
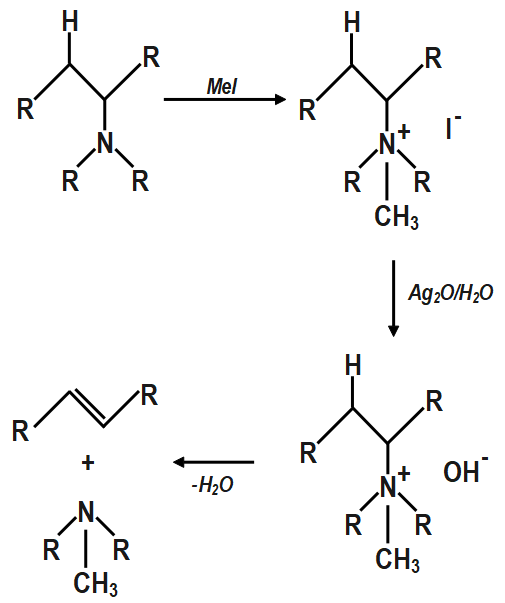
The products which are made is an alkene and a tertiary amine. With addition of silver oxide and water to quaternary ammonium iodide salt and heating of mixture results in the formation of elimination reactions which result in required products.
Thus we know,
\[\left( {a,c} \right)\]
a. The oxidation of \[3\] amine to amine oxide followed by Cope reaction on heating gives Hofmann alkene (less substituted).
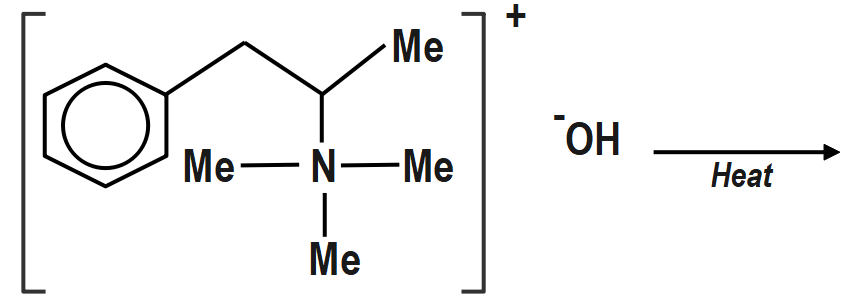
b. \[\left( {II} \right)\;\] will not give Hofmann alkene. Benzylic Hydrogen atom is more acidic due to
\[\left( { - I} \right)\] effect of\[Ph\].
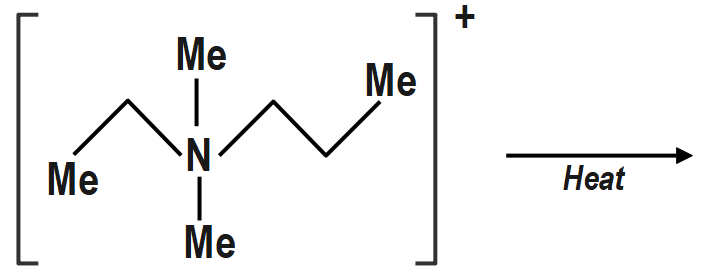
c. \[\left( {III} \right)\] will give Hofmann alkene \[C{H_2} = C{H_2}\]
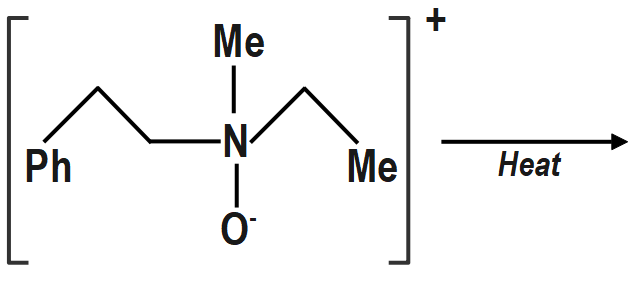
d. \[\left( {IV} \right)\] will not give Hofmann alkene (Cope reaction). Same explanation as in \[\left( b \right)\] above.
Note:
They are mostly gases from two or four carbon atoms, from five to seventeen they are liquids and from eighteen onwards they are solids at room temperature. They are lighter than water and are insoluble in the same. They are soluble in organic solvents. The replacement of the iodine by a hydroxyl anion is followed by an elimination reaction to form the alkene as well.
Complete step by step solution:
It does not have beta hydrogens present which is why methyl iodide is used in excess which thus cannot complete elimination. If there are two different sets of beta hydrogen present in the alkyl group then the alkene isomer having less substituted double bond is made as the major product of the reaction.
The Hofmann rule states that the major alkene product is the least substituted and least stable product when it comes to asymmetrical amines. The Hofmann elimination can be illustrated as follows:

The products which are made is an alkene and a tertiary amine. With addition of silver oxide and water to quaternary ammonium iodide salt and heating of mixture results in the formation of elimination reactions which result in required products.
Thus we know,
\[\left( {a,c} \right)\]
a. The oxidation of \[3\] amine to amine oxide followed by Cope reaction on heating gives Hofmann alkene (less substituted).
b. \[\left( {II} \right)\;\] will not give Hofmann alkene. Benzylic Hydrogen atom is more acidic due to
\[\left( { - I} \right)\] effect of\[Ph\].
c. \[\left( {III} \right)\] will give Hofmann alkene \[C{H_2} = C{H_2}\]
d. \[\left( {IV} \right)\] will not give Hofmann alkene (Cope reaction). Same explanation as in \[\left( b \right)\] above.
Note:
They are mostly gases from two or four carbon atoms, from five to seventeen they are liquids and from eighteen onwards they are solids at room temperature. They are lighter than water and are insoluble in the same. They are soluble in organic solvents. The replacement of the iodine by a hydroxyl anion is followed by an elimination reaction to form the alkene as well.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




