
Which of the following will show GI?
A.

B.
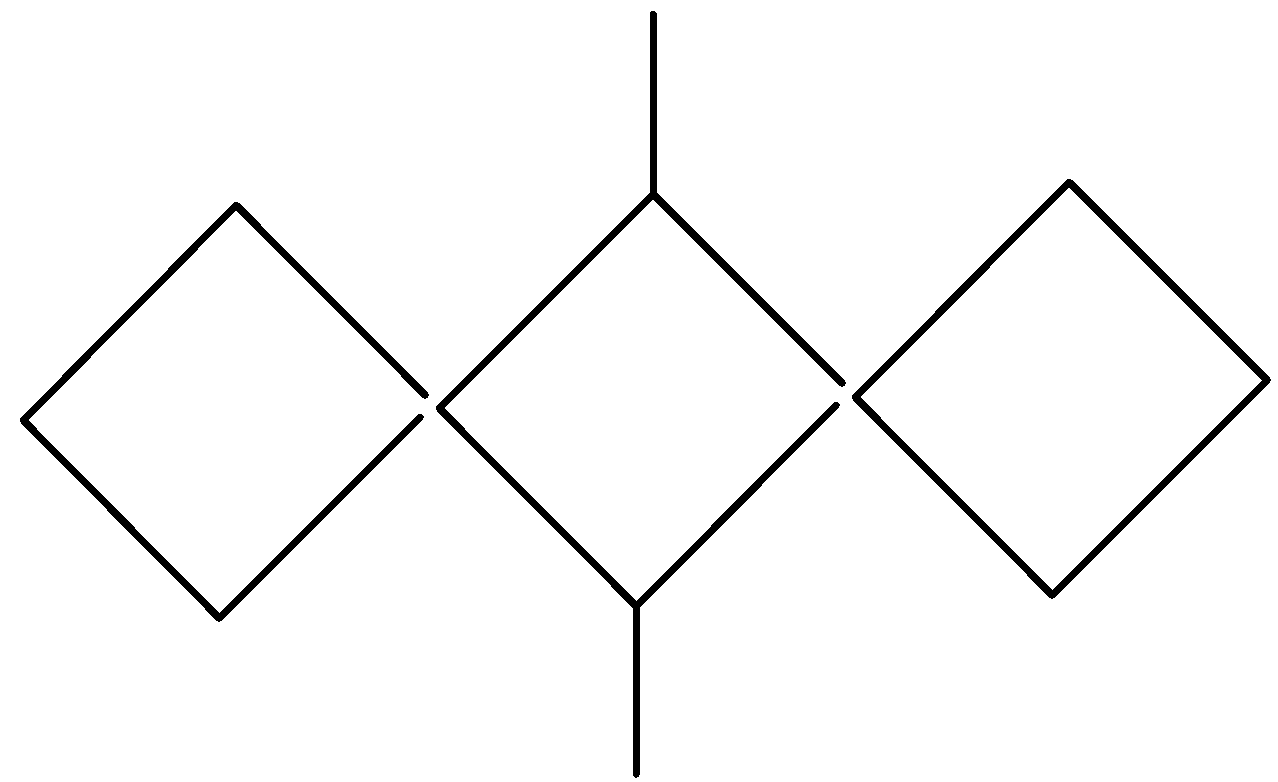
C.
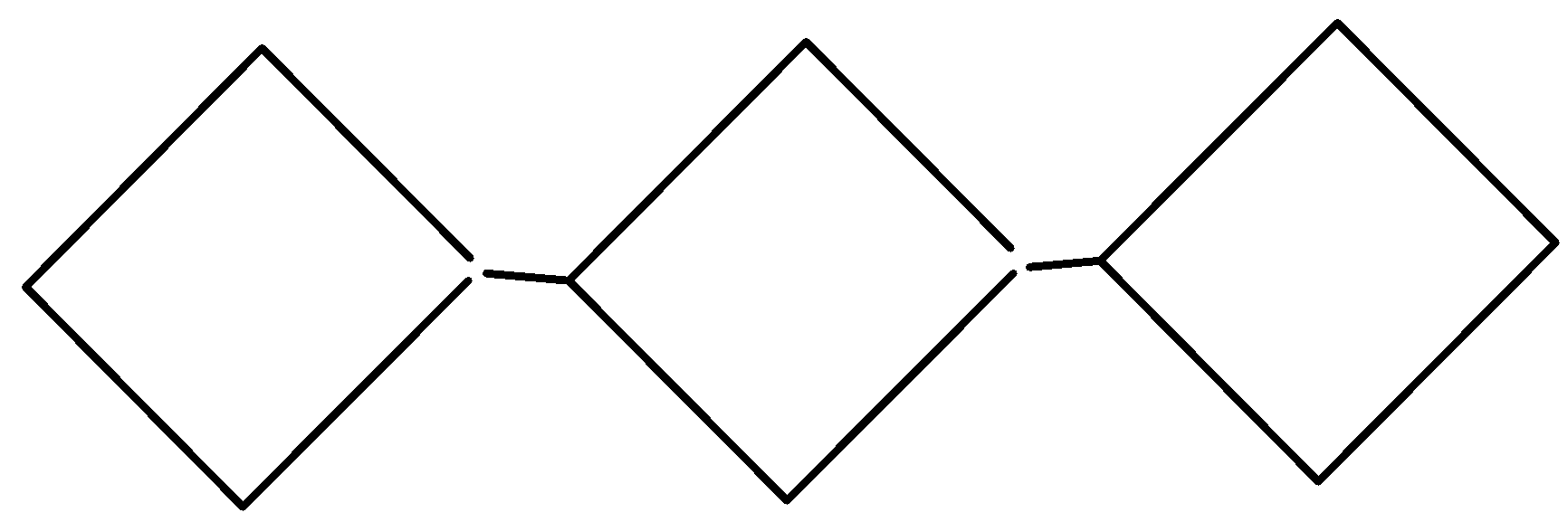
D. All of these



Answer
577.8k+ views
Hint:. For geometrical isomerism to occur, the compound must have at least one double bond of carbon and the groups or atoms that are attached to the double bonded carbon must be different.
Complete step by step answer:
In order to answer our question, let us get to know some facts about isomerism. Two or more compounds having the same molecular formula but different physical and chemical properties are called isomers and this phenomenon is called isomerism. Geometrical isomerism is a type of stereoisomerism. The compounds having the same molecular as well as same structural formulae but differing in the relative arrangement of the atoms or groups in space are called stereoisomers and the phenomenon, stereomerism respectively. In alkenes,the geometrical isomerism is because of restricted or hindered rotation across double bonds of carbon. As a result, the organic compounds of the type ${{C}_{2}}{{A}_{2}}{{B}_{2}}$ with structural formula BAC = CAB represent two spatial arrangements or isomers, named geometrical isomers. If the groups are present on the same side of the double bond, then it is called a cis isomer. Whereas, if it is present on opposite sides, then we call it the trans isomer.
Condition for geometrical isomerism:
A. It must have at least one carbon-carbon double bond in it.
B. The atoms or groups attached to the double bonded carbon must be different.
As we can see that only one option i.e compound of option A satisfies the above conditions. It is the only compound that is an alkene, having double bonds and different groups. So, compound A will show geometrical isomerism and hence our answer is option A.
So, the correct answer is “Option A”.
Note: Following points are to be noted regarding cis and trans isomers:
a. Dipole moment of cis isomer is normally higher than that of trans isomer.
b. The boiling point and melting point of cis isomer is higher than trans isomer.
c. Trans isomers are more stable than cis isomers, due to less repulsion.
Complete step by step answer:
In order to answer our question, let us get to know some facts about isomerism. Two or more compounds having the same molecular formula but different physical and chemical properties are called isomers and this phenomenon is called isomerism. Geometrical isomerism is a type of stereoisomerism. The compounds having the same molecular as well as same structural formulae but differing in the relative arrangement of the atoms or groups in space are called stereoisomers and the phenomenon, stereomerism respectively. In alkenes,the geometrical isomerism is because of restricted or hindered rotation across double bonds of carbon. As a result, the organic compounds of the type ${{C}_{2}}{{A}_{2}}{{B}_{2}}$ with structural formula BAC = CAB represent two spatial arrangements or isomers, named geometrical isomers. If the groups are present on the same side of the double bond, then it is called a cis isomer. Whereas, if it is present on opposite sides, then we call it the trans isomer.
Condition for geometrical isomerism:
A. It must have at least one carbon-carbon double bond in it.
B. The atoms or groups attached to the double bonded carbon must be different.
As we can see that only one option i.e compound of option A satisfies the above conditions. It is the only compound that is an alkene, having double bonds and different groups. So, compound A will show geometrical isomerism and hence our answer is option A.
So, the correct answer is “Option A”.
Note: Following points are to be noted regarding cis and trans isomers:
a. Dipole moment of cis isomer is normally higher than that of trans isomer.
b. The boiling point and melting point of cis isomer is higher than trans isomer.
c. Trans isomers are more stable than cis isomers, due to less repulsion.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




