
Which of the following will not undergo nucleophilic aromatic substitution?
I.
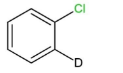
II.
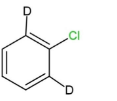
III.
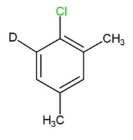
IV.
A.$I,II\,$ and $III$
B.$II$ and $IV$
C. $III$ and $IV$
D. only $IV$



Answer
533.1k+ views
Hint:Nucleophilic substitution is a type of reaction, where a nucleophile takes the place of a leaving group in any organic compound. When a group is removed from the aromatic ring, and the nucleophile gets attached, the reaction is called a nucleophilic aromatic substitution reaction.
Complete step-by-step answer:Any aromatic compound has the activating positions of ortho and para. These ortho and para positions contain a beta- hydrogen that makes them activating. These positions are activated for the incoming nucleophile, and the nucleophilic aromatic substitution reaction occurs. If any group takes the place at ortho and para position and replaces the beta- hydrogen, then the nucleophilic aromatic substitution reaction is not supported in that compound.
We can see that compounds,$I,II\,$and $III$ contain beta- hydrogen, or the deuterium ion, which is an isotope of hydrogen, at the ortho and para positions. So these compounds will easily undergo nucleophilic aromatic substitution.
While we can see that compound $IV$contains methyl groups at all the free ortho and para positions, which is attached by replacing a beta- hydrogen. So, this compound will not undergo nucleophilic aromatic substitution reaction.
Hence, only $IV$will not show nucleophilic aromatic substitution, so option D is correct.
Note:Nucleophilic substitution reactions are of 2 types, nucleophilic substitution 1, ${{S}_{N}}^{1}$ and nucleophilic substitution 2, ${{S}_{N}}^{2}$. Which happens in 2 steps and 1 step respectively. ${{S}_{N}}^{2}$reactions happen with inversion of configuration.
Complete step-by-step answer:Any aromatic compound has the activating positions of ortho and para. These ortho and para positions contain a beta- hydrogen that makes them activating. These positions are activated for the incoming nucleophile, and the nucleophilic aromatic substitution reaction occurs. If any group takes the place at ortho and para position and replaces the beta- hydrogen, then the nucleophilic aromatic substitution reaction is not supported in that compound.
We can see that compounds,$I,II\,$and $III$ contain beta- hydrogen, or the deuterium ion, which is an isotope of hydrogen, at the ortho and para positions. So these compounds will easily undergo nucleophilic aromatic substitution.
While we can see that compound $IV$contains methyl groups at all the free ortho and para positions, which is attached by replacing a beta- hydrogen. So, this compound will not undergo nucleophilic aromatic substitution reaction.
Hence, only $IV$will not show nucleophilic aromatic substitution, so option D is correct.
Note:Nucleophilic substitution reactions are of 2 types, nucleophilic substitution 1, ${{S}_{N}}^{1}$ and nucleophilic substitution 2, ${{S}_{N}}^{2}$. Which happens in 2 steps and 1 step respectively. ${{S}_{N}}^{2}$reactions happen with inversion of configuration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




