
Which of the following will give the maximum number of isomers?
a.) \[{{[Co{{(py)}_{3}}{{(N{{H}_{3}})}_{3}}]}^{3+}}\]
b.) \[{{[Ni(en){{(N{{H}_{3}})}_{4}}]}^{2+}}\]
c.) \[{{[Fe({{C}_{2}}{{O}_{4}}){{(en)}_{2}}]}^{2-}}\]
d.) \[{{[Co{{(N{{O}_{2}})}_{2}}{{(N{{H}_{3}})}_{4}}]}^{+}}\]
Answer
595.8k+ views
Hint: Isomers are the two molecules which have the same molecular formula but differ structurally. Hence, isomers contain the same number of atoms, but the arrangement of atoms differs. Properties of the isomers are going to change due to difference in the structure.
Complete step by step solution:
We have to find which coordination complex forms the maximum numbers of isomers from the given options.
Coming to given options. Option A. \[{{[Co{{(py)}_{3}}{{(N{{H}_{3}})}_{3}}]}^{3+}}\]
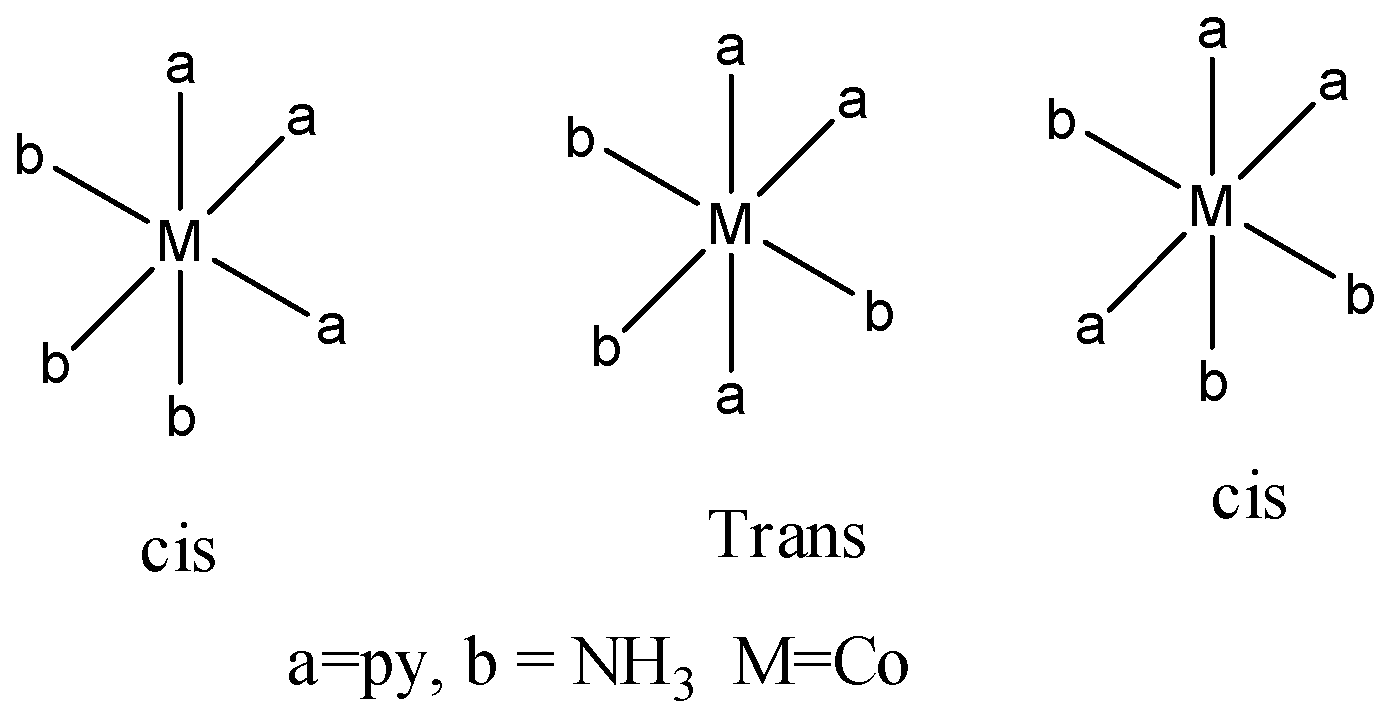
There are three geometrical isomers for option A.
Coming to option B, \[{{[Ni(en){{(N{{H}_{3}})}_{4}}]}^{2+}}\]. This complex won’t show any geometrical isomerism due to presence of ethylene diamine ligand (en). Ethylene diamine is a bidentate ligand. It won’t allow the molecule to rotate freely. So, option B is not the correct answer.
Coming to option C, \[{{[Fe({{C}_{2}}{{O}_{4}}){{(en)}_{2}}]}^{2-}}\]. This complex won’t show any geometrical isomerism due to presence of ethylene diamine ligand (en) and oxalate ligands. Ethylene diamine and oxalate ligands are bidentate ligands. They won’t allow the molecule to rotate freely. So, option C is not the correct answer.
Coming to option D, \[{{[Co{{(N{{O}_{2}})}_{2}}{{(N{{H}_{3}})}_{4}}]}^{+}}\]. It shows geometrical isomerism and linkage isomers. First we discuss geometrical isomerism.
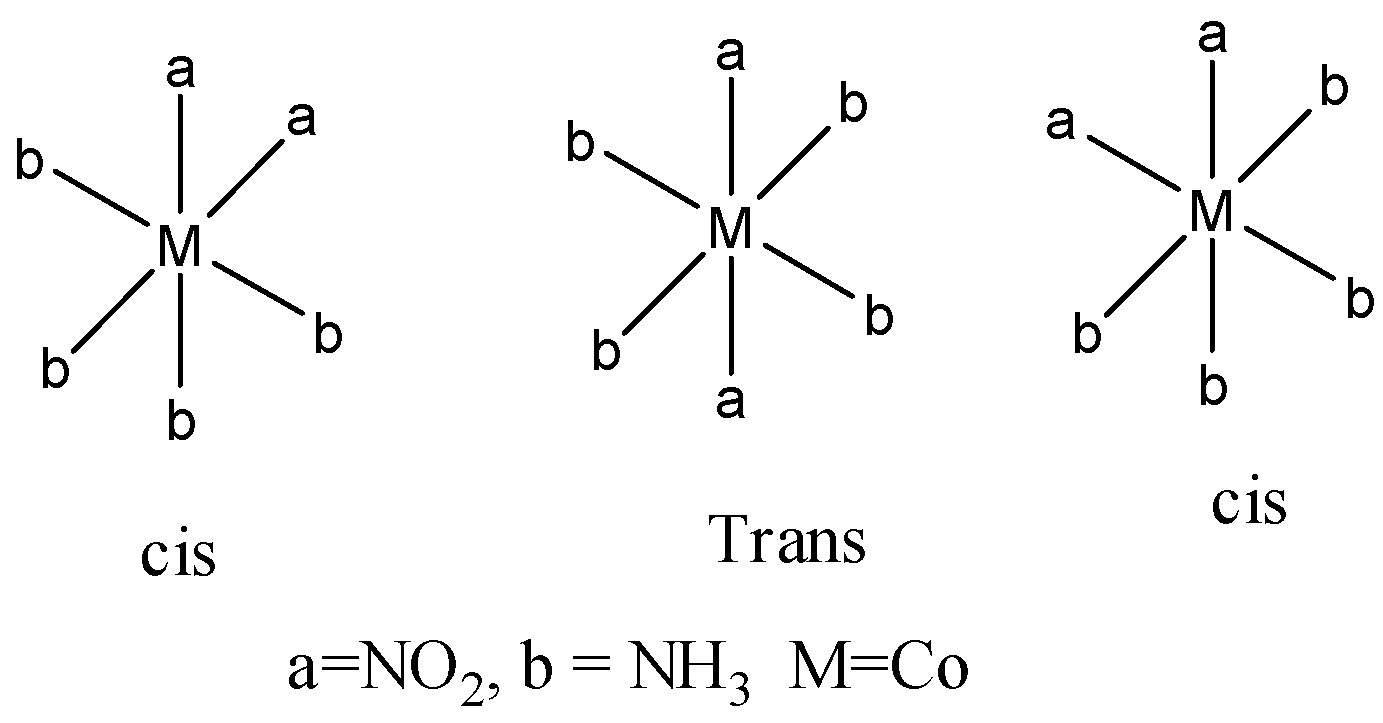
The option D shows three geometrical isomers.
Coming to linkage isomerism.
The Nitro ligand attached to metal in two ways. In the first way through nitrogen it attaches to metal.
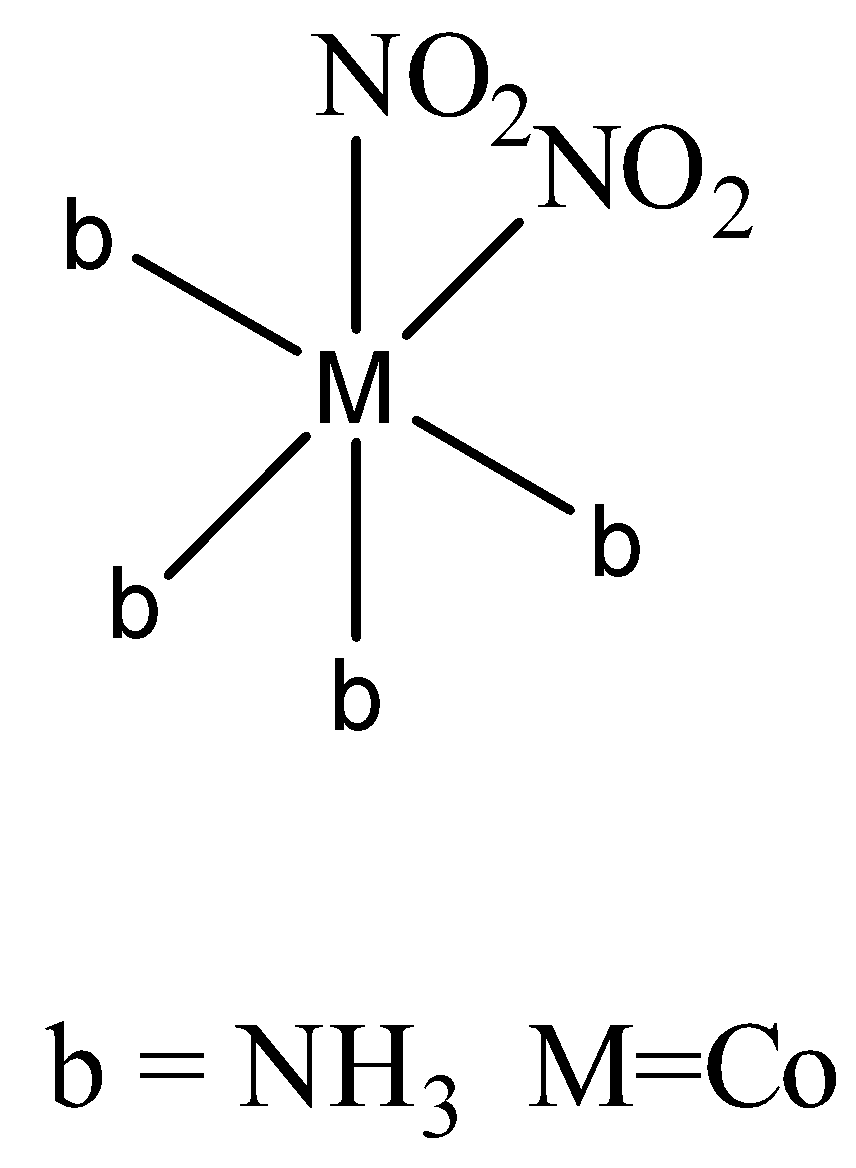
In the second way it attaches through oxygen,
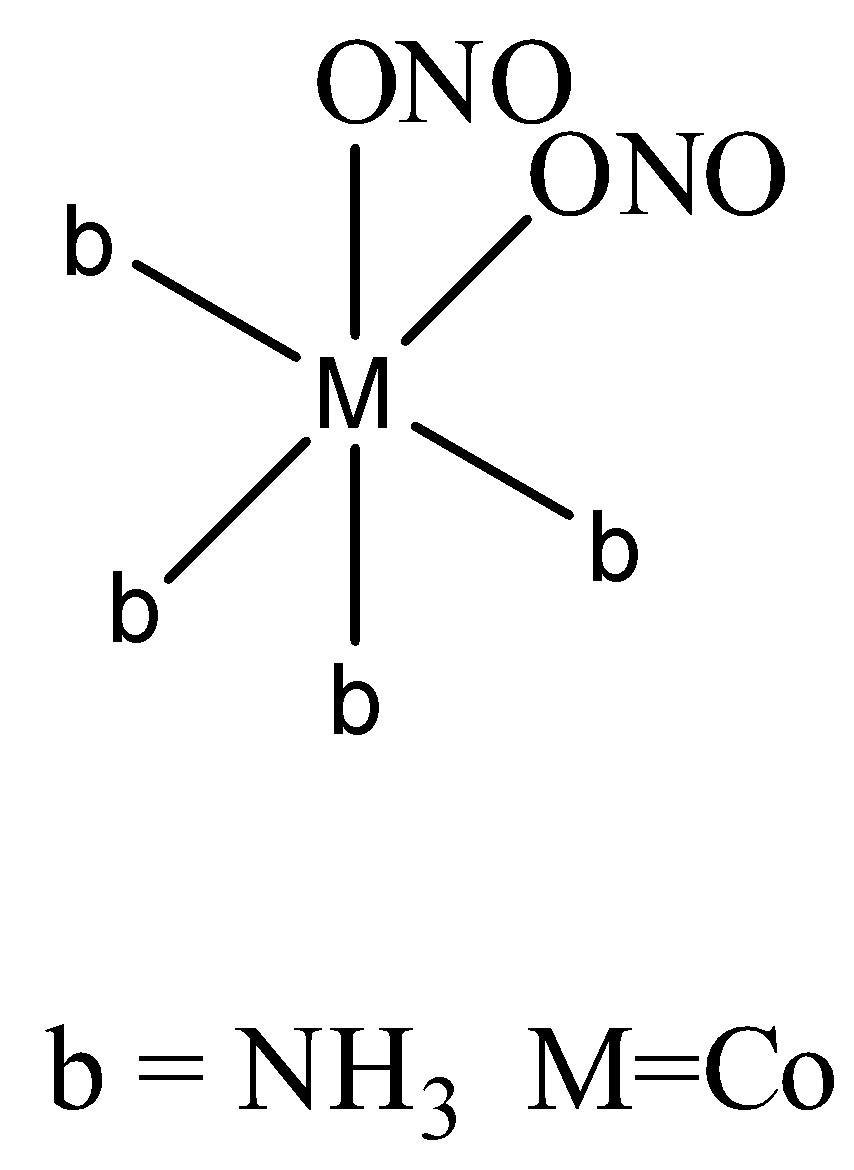
Totally \[{{[Co{{(N{{O}_{2}})}_{2}}{{(N{{H}_{3}})}_{4}}]}^{+}}\] forms five isomers.
There \[{{[Co{{(N{{O}_{2}})}_{2}}{{(N{{H}_{3}})}_{4}}]}^{+}}\]has maximum number of isomers among the given options.
So, the correct option is D.
Note: The possible number of isomers of a given coordination compound is going to depend on the number of geometrical isomers it forms and number of linkage isomers are going to exhibit by the given coordination complex.
Complete step by step solution:
We have to find which coordination complex forms the maximum numbers of isomers from the given options.
Coming to given options. Option A. \[{{[Co{{(py)}_{3}}{{(N{{H}_{3}})}_{3}}]}^{3+}}\]

There are three geometrical isomers for option A.
Coming to option B, \[{{[Ni(en){{(N{{H}_{3}})}_{4}}]}^{2+}}\]. This complex won’t show any geometrical isomerism due to presence of ethylene diamine ligand (en). Ethylene diamine is a bidentate ligand. It won’t allow the molecule to rotate freely. So, option B is not the correct answer.
Coming to option C, \[{{[Fe({{C}_{2}}{{O}_{4}}){{(en)}_{2}}]}^{2-}}\]. This complex won’t show any geometrical isomerism due to presence of ethylene diamine ligand (en) and oxalate ligands. Ethylene diamine and oxalate ligands are bidentate ligands. They won’t allow the molecule to rotate freely. So, option C is not the correct answer.
Coming to option D, \[{{[Co{{(N{{O}_{2}})}_{2}}{{(N{{H}_{3}})}_{4}}]}^{+}}\]. It shows geometrical isomerism and linkage isomers. First we discuss geometrical isomerism.

The option D shows three geometrical isomers.
Coming to linkage isomerism.
The Nitro ligand attached to metal in two ways. In the first way through nitrogen it attaches to metal.

In the second way it attaches through oxygen,

Totally \[{{[Co{{(N{{O}_{2}})}_{2}}{{(N{{H}_{3}})}_{4}}]}^{+}}\] forms five isomers.
There \[{{[Co{{(N{{O}_{2}})}_{2}}{{(N{{H}_{3}})}_{4}}]}^{+}}\]has maximum number of isomers among the given options.
So, the correct option is D.
Note: The possible number of isomers of a given coordination compound is going to depend on the number of geometrical isomers it forms and number of linkage isomers are going to exhibit by the given coordination complex.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




