
Which of the following will be soluble in sodium bicarbonate solution?
A.
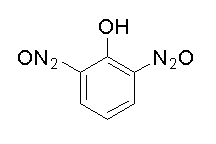
B.
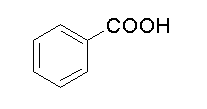
C.
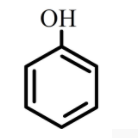
D.
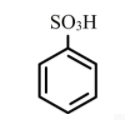




Answer
560.1k+ views
Hint: Solubility of the acid component and base component is dependent on the strength of the acid and base. Sodium bicarbonate is a weak base which reacts with strong organic acids which have pKa range from 4 to 5 liberated carbon dioxide gas.
Complete step by step answer:
The given compound sodium bicarbonate solution is a weak base, it reacts with strong acid to form salt and water by releasing carbon dioxide.
The solubility of strong acid is high in weak base as compared to the solubility of weak acid in weak base.
The given compound 2,6 dinitrophenol is a strong acid, it will dissolve in sodium bicarbonate to form salt and water by liberating carbon dioxide.
The benzene sulphonic acid is a strong acid, therefore it will dissolve in sodium bicarbonate solution to form salt and water by liberating carbon dioxide.
The benzoic acid is also a strong acid, therefore it will dissolve in sodium bicarbonate solution to form salt and water by liberating carbon dioxide..
The compound phenol is considered as a weak acid. Therefore, it will not dissolve in sodium bicarbonate solution and does not release carbon dioxide.
Therefore, the correct option is A, B, and D.
Note:
$NaHC{O_3}$ contains $N{a^ + }$ and $HC{O_3}^ -$ ion. The pKa range of phenol is between 8 to 14 so the bicarbonate is not able to deprotonate the phenol functional group and thus being an acid also phenol does not give any reaction.
Complete step by step answer:
The given compound sodium bicarbonate solution is a weak base, it reacts with strong acid to form salt and water by releasing carbon dioxide.
The solubility of strong acid is high in weak base as compared to the solubility of weak acid in weak base.
The given compound 2,6 dinitrophenol is a strong acid, it will dissolve in sodium bicarbonate to form salt and water by liberating carbon dioxide.
The benzene sulphonic acid is a strong acid, therefore it will dissolve in sodium bicarbonate solution to form salt and water by liberating carbon dioxide.
The benzoic acid is also a strong acid, therefore it will dissolve in sodium bicarbonate solution to form salt and water by liberating carbon dioxide..
The compound phenol is considered as a weak acid. Therefore, it will not dissolve in sodium bicarbonate solution and does not release carbon dioxide.
Therefore, the correct option is A, B, and D.
Note:
$NaHC{O_3}$ contains $N{a^ + }$ and $HC{O_3}^ -$ ion. The pKa range of phenol is between 8 to 14 so the bicarbonate is not able to deprotonate the phenol functional group and thus being an acid also phenol does not give any reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




