
Which of the following will be obtained by keeping ether in contact with air for a long time:
A. \[{C_2}{H_5} - O - CH\left( {C{H_3}} \right) - O - OH\]
B. \[{C_2}{H_5} - OC{H_3} - OH\]
C. \[{C_2}{H_5} - O - {C_2}{H_5}OH\]
D. \[C{H_3} - O - CH\left( {C{H_3}} \right) - O - OH\]
Answer
373.5k+ views
Hint: When ethers are exposed to air and light for a long time, they get oxidised to form hydroperoxides or simply peroxides. It is a free radical reaction.
Complete Step by Step Answer:
The substitution of a hydrogen atom of an aliphatic or aromatic hydrocarbon by an alkoxy (OR) or aryloxy (OAr) group gives ethers.
It may also be regarded as the group of compounds obtained by substituting the hydrogen atom of alcohol or a phenol with an alkyl or an aryl group. The general formula of ether is R-O-R, R-O-Ar, R-O-R’, and Ar-O-Ar, where R, and R’ are the alkyl groups and Ar is an aryl group.
Ether on reaction with air and light for a long time gets converted to peroxides. It is a free radical reaction and oxidation occurs at the carbon atom next to the ethereal oxygen to form hydroperoxides.
This reaction happens in three steps.
1. It involves the attack of the alkoxy free radical on the carbon atom next to the ethereal oxygen leading to the formation of ether free radical.
This is the chain initiation step.
2. Ether free radical then attacks the molecular oxygen free radical. As a result, the oxygen atoms get attached to the reactant molecule.
3. Ether peroxide is formed in the last step by the abstraction of hydrogen-free radicals.
These two are chain propagation steps.
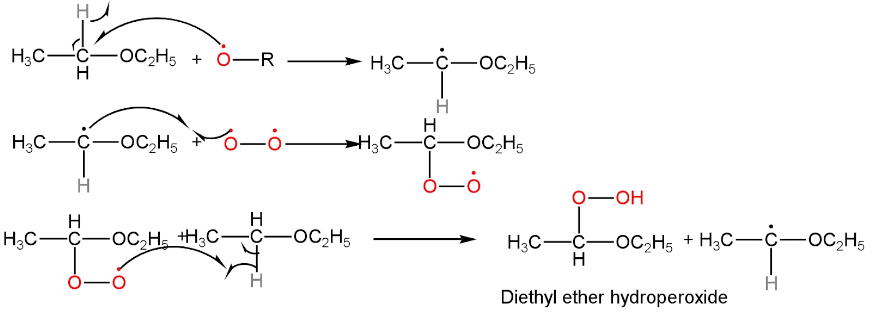
Image: Mechanism of reaction of ether with air and light.
The chemical formula of diethyl ether hydroperoxide is represented by option A.
So, option A is correct.
Note: Ether is susceptible to light and air, and will form flammable peroxides. Ether peroxides have an increased boiling point than ether and in a dry state are explosives. Commercial diethyl ether is commonly equipped with trace quantities of the antioxidant butylated hydroxytoluene which decreases the yield of peroxides.
Complete Step by Step Answer:
The substitution of a hydrogen atom of an aliphatic or aromatic hydrocarbon by an alkoxy (OR) or aryloxy (OAr) group gives ethers.
It may also be regarded as the group of compounds obtained by substituting the hydrogen atom of alcohol or a phenol with an alkyl or an aryl group. The general formula of ether is R-O-R, R-O-Ar, R-O-R’, and Ar-O-Ar, where R, and R’ are the alkyl groups and Ar is an aryl group.
Ether on reaction with air and light for a long time gets converted to peroxides. It is a free radical reaction and oxidation occurs at the carbon atom next to the ethereal oxygen to form hydroperoxides.
This reaction happens in three steps.
1. It involves the attack of the alkoxy free radical on the carbon atom next to the ethereal oxygen leading to the formation of ether free radical.
This is the chain initiation step.
2. Ether free radical then attacks the molecular oxygen free radical. As a result, the oxygen atoms get attached to the reactant molecule.
3. Ether peroxide is formed in the last step by the abstraction of hydrogen-free radicals.
These two are chain propagation steps.

Image: Mechanism of reaction of ether with air and light.
The chemical formula of diethyl ether hydroperoxide is represented by option A.
So, option A is correct.
Note: Ether is susceptible to light and air, and will form flammable peroxides. Ether peroxides have an increased boiling point than ether and in a dry state are explosives. Commercial diethyl ether is commonly equipped with trace quantities of the antioxidant butylated hydroxytoluene which decreases the yield of peroxides.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

What is the median of the first 10 natural numbers class 10 maths CBSE




