
Which of the following tests can be used to distinguish between two isomeric ketones: 3-pentanone and 2-pentanone?
(A) ${I_2}/NaOH$
(B) $NaS{O_3}H$
(C) $NaCN/HCl$
(D) $2,4 - DNP$
Answer
578.7k+ views
Hint: Ketones can be distinguished by two chemical tests namely iodoform test and 2,4-DNP test. 2,4 – DNP test is done for identifying carbonyl compounds on the whole but iodoform test is performed to distinguish between different ketones as well.
Complete step by step answer:
3-pentanone and 2-pentanone are both ketones having five carbon atoms in their structure. The numbers 2 and 3 refers to the presence of the carbonyl group $ - C = O$ on either the 2nd or 3rd carbon atom of the ketone. To distinguish between these two ketones, iodoform tests can be performed.
Iodoform test: Reaction of carbonyl compounds with iodine in the presence of an alkali (NaOH).
Take 2-pentanone $\left( {{H_3}C - C = O - C{H_2} - C{H_2} - C{H_3}} \right)$ and react with iodine $\left( {{I_2}} \right)$ in NaOH medium, in the first step, the alpha hydrogen atoms of the methyl $\left( {C{H_3}} \right)$ group gets substituted by the iodine atoms. This is the nucleophilic substitution reaction. In the next step, by the attack of $O{H^ - }$ ion on the carbon atom, the $C - C{I_3}$ bond breaks to form $CH{I_3}$ (triiodomethane) which is lemon yellow colored precipitate. Triiodomethane is also called iodoform. This reaction occurred due to the presence of a methyl group on 2-pentanone.
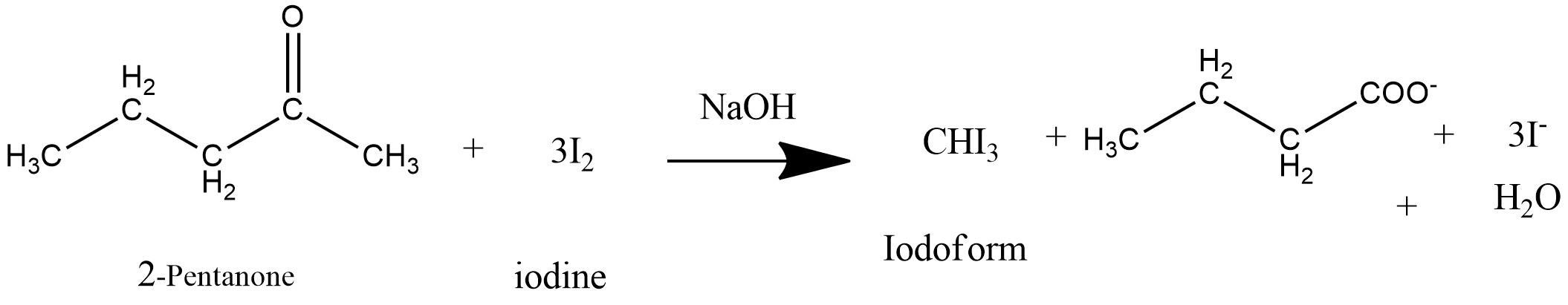
Now take 3-pentanone $\left( {{H_3}C - {H_2}C\left( {C = O} \right)\left( {C{H_2}C{H_3}} \right)} \right)$and add few drops of iodine $\left( {{I_2} + {\kern 1pt} KI} \right)$ and NaOH solution, no yellow colored precipitate is formed. Thus it is proved that 3-pentanone does not give iodoform test whereas 2-pentanone a methyl ketone gives this test positive.
So, the correct answer is “Option A”.
Note: Iodoform test is generally used to determine the presence of methyl group on the adjacent carbon of the functional group attached carbon atom. This means an iodoform test is performed to distinguish methyl ketones from all the other ketones.
Complete step by step answer:
3-pentanone and 2-pentanone are both ketones having five carbon atoms in their structure. The numbers 2 and 3 refers to the presence of the carbonyl group $ - C = O$ on either the 2nd or 3rd carbon atom of the ketone. To distinguish between these two ketones, iodoform tests can be performed.
Iodoform test: Reaction of carbonyl compounds with iodine in the presence of an alkali (NaOH).
Take 2-pentanone $\left( {{H_3}C - C = O - C{H_2} - C{H_2} - C{H_3}} \right)$ and react with iodine $\left( {{I_2}} \right)$ in NaOH medium, in the first step, the alpha hydrogen atoms of the methyl $\left( {C{H_3}} \right)$ group gets substituted by the iodine atoms. This is the nucleophilic substitution reaction. In the next step, by the attack of $O{H^ - }$ ion on the carbon atom, the $C - C{I_3}$ bond breaks to form $CH{I_3}$ (triiodomethane) which is lemon yellow colored precipitate. Triiodomethane is also called iodoform. This reaction occurred due to the presence of a methyl group on 2-pentanone.
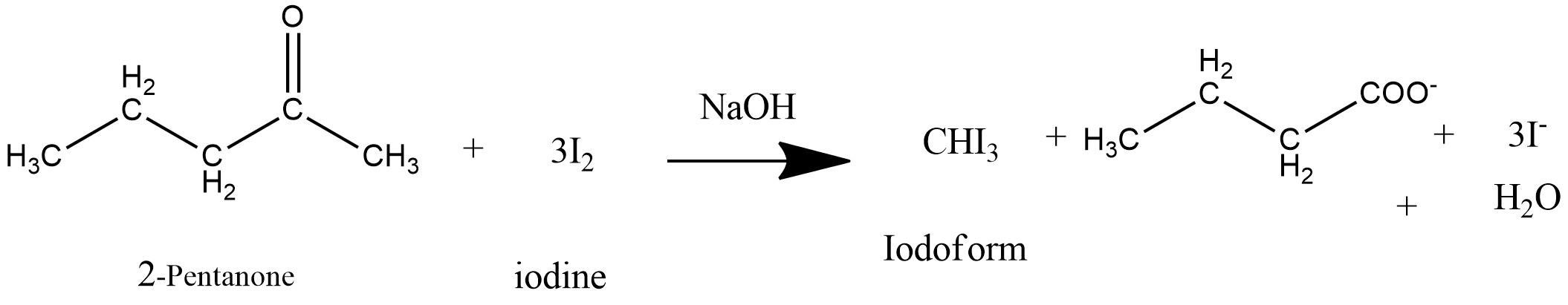
Now take 3-pentanone $\left( {{H_3}C - {H_2}C\left( {C = O} \right)\left( {C{H_2}C{H_3}} \right)} \right)$and add few drops of iodine $\left( {{I_2} + {\kern 1pt} KI} \right)$ and NaOH solution, no yellow colored precipitate is formed. Thus it is proved that 3-pentanone does not give iodoform test whereas 2-pentanone a methyl ketone gives this test positive.
So, the correct answer is “Option A”.
Note: Iodoform test is generally used to determine the presence of methyl group on the adjacent carbon of the functional group attached carbon atom. This means an iodoform test is performed to distinguish methyl ketones from all the other ketones.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




